These datapoints represent the challenge and opportunity that medical technology firms have in the future.
May 9, 2013
|
Stacy Enxing Seng, President of Vascular Therapies, Covidien |
The IBF MedTech Investing Conference, which takes place in Minneapolis annually, has been in recent years an exercise in complaining about the FDA. Add to that a sense doom and gloom given the shrinking pool of venture capital available for investment in medtech.
This year, however, there was less FDA bashing, and the conference - held Wednesday - had an optimistic tone. Kevin Wasserstein, an affiliate director at Versant Ventures, reported being told that there have been "more success stories with the FDA than we have seen for a long time" and that "the stage is set for an opening of the IPO window."
All very heartening of course.
However, the keynote speaker at lunch, in a deft and substantive presentation underlined the significant challenges (and thereby opportunities) for the industry. Stacy Enxing Seng, president of Covidien's vascular therapies business, addressed how the industry needs to adapt. Two slides from her presentation are worth further attention.
|
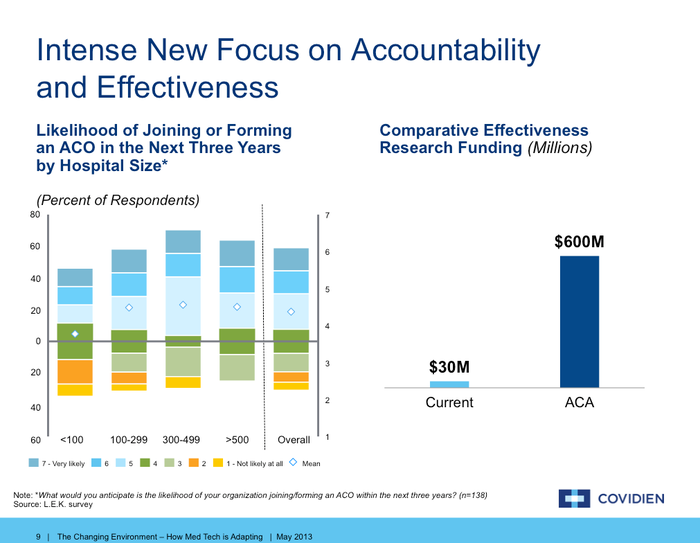
Look at the slivers that show the small percentage of hospitals that say they are not likely to join an ACO in the next three years. That essentially means that all those expensive sales people device firms have hired to persuade physicians to buy their widget over a competitor's product is all for naught. Those physicians will have less and less say moving forward. The ACO will be the purchasing center and that entity is driven by desire to control cost and improve outcomes.
The right side of the slide shows how important comparative effectiveness research is going to be in the near future. Under the ACO model, $600 million is going to be pumped in to this enterprise to prove whether new products are more effective in caring for patients than standard models of care. This is all the more important if the new product device firms are touting happen to be more expensive than current models of treatment.
And here is the next slide:
|
This slide should make device firm executives hang their heads in shame. Hospital customers are basically saying vendors are largely not meeting their needs across a wide spectrum of priorities at the hospital.
Going forward device firms need to understand that their fortunes are tied to that of their customers, and that sharing risk with hospitals is inevitable. Those who heed that and adapt will find their pot of gold.
-- By Arundhati Parmar, Senior Editor, MD+DI
Related Content
Is Medtronic Interested in Investing in Early Stage Companies? Well, Not Really
The Three Cs That Can Get Your Next MedTech Venture Funded
You May Also Like