November 29, 2012
 Full-contract manufacturers enable medical device OEMs to achieve less-complex project management, according to Kevin Allison, business development manager at Crescent Industries Inc. (New Freedom, PA).
Full-contract manufacturers enable medical device OEMs to achieve less-complex project management, according to Kevin Allison, business development manager at Crescent Industries Inc. (New Freedom, PA).
MPMN: What technical and logistical benefits does the medical device OEM derive from working with a full-contract manufacturer?
Allison: One benefit that the OEM gains by working with a full-contract manufacturer is less-complex project management. When a medical device OEM partners with a contract manufacturer, the supplier holds the responsibility for the project from beginning to end. As a result of this relationship, medical device products achieve speedier time to market. Another benefit that OEMs derive from partnering with contract manufacturers is that they can collaborate on all manufacturability issues, from design and prototyping to product verification and performance. By working with a single company starting with the design phase, the OEM can ensure that the contract manufacturer adheres to design principles and best practices, enabling the partners to ensure design continuity and control over the course of the manufacturing process. Consequently, the OEM can be certain that components will meet specifications and expectations.
MPMN: What technical expertise should a medical device OEM demand of a full-contract manufacturer?
Allison: The medical device OEM should demand that a full-contract manufacturer adhere to such standards as ISO 13485:2003 and CGMP guidelines. Along with the ISO quality certifications, contract manufacturers should be able to provide consistent and repeatable products by utilizing scientific and decoupled injection molding processes. In addition, contract manufacturers should ensure that their processes are validated so that the OEM's products meet the required qualification and validation procedures. These validation processes are beneficial to the OEM because they help prevent the manufacture of products that do not meet specifications.
MPMN: How are the demands on full-contract manufacturers changing, and what should OEMs and their partners do to keep pace with changing standards and technologies?
Allison: With increased emphasis on production qualification and validation, the OEM must ensure that the full-contract manufacturer acquire upfront information about the requirements, end use, and classification of the product. With an understanding of these expectations and a scientific approach to quality solutions, the supplier can identify key variables so that products are manufactured consistently to OEM expectations. These quality solutions include installation qualification, operational qualification, performance qualification, capabilities study, gage repeatability and reproducibility, process failure mode effects analysis, and control plans.
Manufacturing and cleanroom device assembly services A full-service contract manufacturer offers injection molding, radio-frequency welding, silk-screen printing, solvent bonding and leak testing, and tipping and punching services. Operating an ISO 9001:2000- and ISO 13485:2003-certified production facility, Angiplast Pvt. Ltd. also performs physicochemical, sterility, pyrogen, and toxicity testing in an in-house laboratory to applicable USP and ISO standards. Additional capabilities include EtO sterilization. Most of the company's medical devices carry the CE mark. The supplier develops production processes, manufactures custom products to specification in short runs and mass-production volumes, and packages medical devices in pouches or kits.
A full-service contract manufacturer offers injection molding, radio-frequency welding, silk-screen printing, solvent bonding and leak testing, and tipping and punching services. Operating an ISO 9001:2000- and ISO 13485:2003-certified production facility, Angiplast Pvt. Ltd. also performs physicochemical, sterility, pyrogen, and toxicity testing in an in-house laboratory to applicable USP and ISO standards. Additional capabilities include EtO sterilization. Most of the company's medical devices carry the CE mark. The supplier develops production processes, manufactures custom products to specification in short runs and mass-production volumes, and packages medical devices in pouches or kits.
Angiplast Pvt. Ltd.
AHMADABAD, INDIA
Contract manufacturing of finished products A full-service supplier of comprehensive engineering, manufacturing, and assembly solutions, ISO 13485-certified and FDA-registered Inteprod LLC partners with medical device makers to develop and produce electromechanical devices, diagnostic systems, and disposables. Its service range comprises transfer to manufacturing, quality assurance and compliance, materials sourcing, production planning, manufacturing control, and finished-product assembly. The company's multidisciplinary technical staff provides long-term sustaining engineering to identify, support, and implement design changes, engineering upgrades, and product and process quality and cost improvements. Fully integrated manufacturing solutions entail product safety elements, product and process verification, design for manufacturing, design for cost reduction, and process control documentation.
A full-service supplier of comprehensive engineering, manufacturing, and assembly solutions, ISO 13485-certified and FDA-registered Inteprod LLC partners with medical device makers to develop and produce electromechanical devices, diagnostic systems, and disposables. Its service range comprises transfer to manufacturing, quality assurance and compliance, materials sourcing, production planning, manufacturing control, and finished-product assembly. The company's multidisciplinary technical staff provides long-term sustaining engineering to identify, support, and implement design changes, engineering upgrades, and product and process quality and cost improvements. Fully integrated manufacturing solutions entail product safety elements, product and process verification, design for manufacturing, design for cost reduction, and process control documentation.
Inteprod LLC
EAGLEVILLE, PA
Electronic manufacturing services Suntron Corp. provides integrated, full-contract electronic manufacturing services and embedded computing systems. The company's capabilities include box build of Class I to Class III devices, PCB assembly, and large-scale system integration. In addition to ultrasound and endoscopy systems, cancer monitoring and diagnostic systems, neurostimulation devices, electrosurgical devices, orthopedic surgical devices, and other safety-critical products, the service provider produces patient monitoring and connectivity systems and hospital asset tracking systems. Class 10,000 and Class 100,000 cleanroom installations enable the FDA-registered and ISO 13485-certified company to produce critical assemblies that meet medical device industry standards.
Suntron Corp. provides integrated, full-contract electronic manufacturing services and embedded computing systems. The company's capabilities include box build of Class I to Class III devices, PCB assembly, and large-scale system integration. In addition to ultrasound and endoscopy systems, cancer monitoring and diagnostic systems, neurostimulation devices, electrosurgical devices, orthopedic surgical devices, and other safety-critical products, the service provider produces patient monitoring and connectivity systems and hospital asset tracking systems. Class 10,000 and Class 100,000 cleanroom installations enable the FDA-registered and ISO 13485-certified company to produce critical assemblies that meet medical device industry standards.
Suntron Corp.
PHOENIX
Diagnostic and drug-delivery device manufacturing services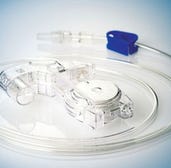 A contract manufacturer of medical diagnostic and drug-delivery devices, Medisize helps select appropriate materials, manufacturing processes, and packaging for OEM customers. Its turnkey competencies extend across several disciplines, such as product design and development, in-house toolmaking, injection molding, insert molding, silicone molding, injection blow molding, film welding, assembly, packaging, and sterilization. All manufacturing is performed in Class 10,000 cleanroom facilities housing 132 injection molding machines and 27 injection blow molding machines.
A contract manufacturer of medical diagnostic and drug-delivery devices, Medisize helps select appropriate materials, manufacturing processes, and packaging for OEM customers. Its turnkey competencies extend across several disciplines, such as product design and development, in-house toolmaking, injection molding, insert molding, silicone molding, injection blow molding, film welding, assembly, packaging, and sterilization. All manufacturing is performed in Class 10,000 cleanroom facilities housing 132 injection molding machines and 27 injection blow molding machines.
Medisize
NÜRENSDORF, SWITZERLAND
Full-contract manufacturing of Class I, II, and III devices Certified to ISO 13485:2003 standards, a full-contract manufacturer performs custom injection molding services in an ISO Class 8 cleanroom and assembly and packaging services in an ISO Class 7 cleanroom. Crescent Industries Inc.'s molding area accommodates up to five vertical/vertical or horizontal injection molding machines that are used to manufacture Class I, II, and III medical devices, including surgical equipment and disposables. Relying on automation, scientific injection molding, and lean manufacturing principles, the company also offers design and development, mold-building, and value-added services.
Certified to ISO 13485:2003 standards, a full-contract manufacturer performs custom injection molding services in an ISO Class 8 cleanroom and assembly and packaging services in an ISO Class 7 cleanroom. Crescent Industries Inc.'s molding area accommodates up to five vertical/vertical or horizontal injection molding machines that are used to manufacture Class I, II, and III medical devices, including surgical equipment and disposables. Relying on automation, scientific injection molding, and lean manufacturing principles, the company also offers design and development, mold-building, and value-added services.
Crescent Industries Inc.
NEW FREEDOM, PA
About the Author(s)
You May Also Like


