May 18, 2015
Create your own user feedback survey
1. ProPlate (Professional Plating Inc.; Booth #2367)
 Torq-Lok is a unique technology applied to braided catheters for enhanced performance. Preliminary testing of Torq-Lok compared to noncoated braids demonstrated a range of 30-66% greater torque response, a 66% increase in torque to failure, and a 90° twist increase. Torq-Lok also maintains flexibility, increases kink resistance, improves pushability, and constrains radial forces while minimizing elongation. The need for annealing the braid ends after manufacturing is eliminated because the process fuses them together preventing fraying. (Braids used in test: 4 French OD, 60 pic count, 12 inches long, 2-over-2-under-2 pattern, 0.001 x 0.004-in. wire. Results may vary.)
Torq-Lok is a unique technology applied to braided catheters for enhanced performance. Preliminary testing of Torq-Lok compared to noncoated braids demonstrated a range of 30-66% greater torque response, a 66% increase in torque to failure, and a 90° twist increase. Torq-Lok also maintains flexibility, increases kink resistance, improves pushability, and constrains radial forces while minimizing elongation. The need for annealing the braid ends after manufacturing is eliminated because the process fuses them together preventing fraying. (Braids used in test: 4 French OD, 60 pic count, 12 inches long, 2-over-2-under-2 pattern, 0.001 x 0.004-in. wire. Results may vary.)
With the application of Torq-Lok, the braid pic count can be reduced. Torq-Lok achieves the same or higher torque response, torque to failure, and twist range. Torq-Lok also maintains the braid wire profile and flexibility. Cost savings for the braid manufacturer can be achieved by reducing material costs and production time. Torq-Lok will also eliminate unraveling at catheter ends, thus reducing additional product and productivity losses. For surgeons, greater device control can result in more accurate placement of self expanding stents or heart valves and potentially less time to complete the procedure, therefore reducing risk to the patient and decreasing costs for the surgeon.
2. Rapid Inspection LLC (Booth #209)
IRIS is a patented 3-D inspection system used to produce AS9102 inspection reports and 3-D color maps of injection molded parts in 48 hours or less.
The process is designed to reduce tool qualification time. Manual inspection can take two weeks or longer for an 8-cavity mold or longer. Rapid Inspection can cut this to 48 hours.
The technology works by encasing an injection-molded part in an epoxy compound. When cured, they are placed in IRIS machines and milled a micro layer at a time. With each pass, a high-resolution camera captures an image and produces a point cloud. From this accurate point cloud, every dimension and 3-D profile can be captured using IRIS software.
CT scanning is the only competing technology and is substantially more expensive than IRIS, according to Rapid Inspection.
3. MTD Micro Molding (Booth #875)
MTD Micro Molding can mold multiple micro components together at once. This technology, launched in late 2014, can improve medical device companies' drug-delivery cannulas.
Traditional manufacturing methods for the development of cannulas have used the heat-forming process of converting fluoropolymer extrusions to cannulas. Next-generation products could follow the new advances that MTD is enabling by instead using polypropylene for cannula products. In terms of technical execution, it is difficult to make a long, thin, straight part such as a cannula. The challenge lies in the fact that for a 0.005-in. wall thickness to flow long-distance, a tiny, delicate core pin must be centered precisely in the cavity. With long length of thin flow, it is impossible to fill an annular ring if material flow becomes uneven. When flow becomes unbalanced, a hydraulic effect is created on one side and pushes the core pin, which produces an ultra-thin wall on the opposite side. This, in turn, causes fill problems and distortion. Precise control over the position of the core is critical, which is why highly capable tooling and molding machines are required to successfully mold a thin-walled cannula for drug delivery. This control makes molding walls as thin as 0.03 mm possible at MTD (see above image).
 4. Martech Medical Products Inc. (Booth #1421)
4. Martech Medical Products Inc. (Booth #1421)

Martech Medical Products has developed the C3 Wave: an FDA approved, wireless, UL certified PICC (peripherally-inserted central catheter) tip location system available in the United States since 2015.
C3 Wave works like other PICC tip location systems, except that it uses a Bluetooth connection to transmit a continuous display of electrocardiograph [ECG] waveform from the C3 hub to an iPad using the C3 Wave App, which will be available on the iTunes store. The C3 Wave App also allows the operator to view and record changes to the ECG waveform as the tip of the catheter approaches the heart.
The device is designed to help ensure proper PICC placement before the patient reaches radiology for an x-ray, minimizing the need to adjust or replace the PICC if it's in the wrong location.
The system was designed, developed, and produced by the team at Martech Medical Products, Inc. Its mechanical and electrical engineers worked together to make C3 Wave an intuitive PICC tip location system. C3 Wave is said to be so user friendly that smartphone users and non-users alike will have no trouble learning how to use the new interface.
5. EG-GILERO (Booth #1429)
 EG-GILERO developed a Local Anesthetic Delivery System for Anutra Medical that launched in 2015. The system consists of a multi-use dispenser, sterile multi-dose cassette, and a sterile single-use syringe. A vial of lidocaine and a prefilled syringe containing sodium bicarbonate are attached to the cassette and then loaded into the dispenser. The syringe is attached to the valve at the front of the dispenser and dispenses specifically measured buffered lidocaine into the syringe. The syringe allows for aspiration and has integral haptic and audible feedback to aid in anesthetic dispense and delivery to the patient.
EG-GILERO developed a Local Anesthetic Delivery System for Anutra Medical that launched in 2015. The system consists of a multi-use dispenser, sterile multi-dose cassette, and a sterile single-use syringe. A vial of lidocaine and a prefilled syringe containing sodium bicarbonate are attached to the cassette and then loaded into the dispenser. The syringe is attached to the valve at the front of the dispenser and dispenses specifically measured buffered lidocaine into the syringe. The syringe allows for aspiration and has integral haptic and audible feedback to aid in anesthetic dispense and delivery to the patient.
The onset of buffered lidocaine is up to 90% faster than unbuffered lidocaine, and the injections are less painful. Inherently, buffered lidocaine is unstable over relatively short periods of time, thus the two drugs must be mixed at the point of care. This system allows clinicians to efficiently buffer lidocaine at the patient's side. By using buffered lidocaine, clinicians can perform procedures immediately following administration of anesthetic. This reduces procedure time, improving clinical throughput.
6. TissueGen Inc. (Booth #1581)
 TissueGen's ELUTE fibers, first commercially available in 2013, deliver a wide range of drugs and therapeutic agents from small pharmaceuticals to proteins (growth factors, enzymes, even viral particles) with retained biological activity directly to the surgical implant site. This patented drug delivery platform enhances the effectiveness of both the drug and provides a mechanical scaffold. ELUTE fiber's novel approach has far-reaching implications for many medical applications including, but not limited to, regenerative medicine, peripheral nerve and spinal cord repair, cardiovascular, orthopedic and dental applications, tumor remediation, and dermal wound healing.
TissueGen's ELUTE fibers, first commercially available in 2013, deliver a wide range of drugs and therapeutic agents from small pharmaceuticals to proteins (growth factors, enzymes, even viral particles) with retained biological activity directly to the surgical implant site. This patented drug delivery platform enhances the effectiveness of both the drug and provides a mechanical scaffold. ELUTE fiber's novel approach has far-reaching implications for many medical applications including, but not limited to, regenerative medicine, peripheral nerve and spinal cord repair, cardiovascular, orthopedic and dental applications, tumor remediation, and dermal wound healing.
TissueGen R&D applications have included:
o Anti-fibrillation devices.
o Cardiovascular stents.
o Coatings for cardiac stents.
o Growth factor-loaded "smart" sutures.
o Nicotine-induced angiogenesis.
o Ocular drug delivery for diabetic retinopathy.
o Scaffolding for peripheral nerve regeneration.
o Urological stents.
o VEGF delivery to induce angiogenesis in the retina.
o Tumor remediation.
o Root canal applications.
o Nerve regeneration.
7. SafeStart Systems LLC (Booth #2166)
 The SafetyGate Pro Med prevents restart hazards from a machine or tool starting without warning after a power loss, breaker trip or accidental unplugging from the wall outlet, in accordance with new IEC 60601 v.3 standards for OEM ME restart protection. By incorporating the circuitry into the machine or tools power cord, ME manufacturers can instantly implement the mandated safety feature without any modifications to the existing housing, structure or ergonomics of their current devices. Preventing unnecessary hazards in the workplace benefits the physicians, patients and ME work environment. The low cost and miniaturization helps ME manufacturers meet FDA and IEC standards at a minimum cost and footprint.
The SafetyGate Pro Med prevents restart hazards from a machine or tool starting without warning after a power loss, breaker trip or accidental unplugging from the wall outlet, in accordance with new IEC 60601 v.3 standards for OEM ME restart protection. By incorporating the circuitry into the machine or tools power cord, ME manufacturers can instantly implement the mandated safety feature without any modifications to the existing housing, structure or ergonomics of their current devices. Preventing unnecessary hazards in the workplace benefits the physicians, patients and ME work environment. The low cost and miniaturization helps ME manufacturers meet FDA and IEC standards at a minimum cost and footprint.
8. Tego (Booth #2049)
Tego's UHF RFID technology has proven to be radiation proof, remaining fully functional after exposure to gamma radiation, eBeam, two different temperatures of autoclave, and chemical agents most commonly found in medical device manufacturing.
Four independent medical device and medical device sterilization companies tested TegoChip's survivability and functionality through four different kinds of medical grade sterilization processes.
The first study exposed 110 Tego inlays to three rounds of gamma sterilization. Each round of gamma sterilization provided between 27 to 33 kGy, such that total exposure to each inlay after all three doses totaled between 81 and 99 kGy. Results indicated that all 110 Tego inlays remained fully functional after three sterilization doses. There were no bit errors detected in over 36 million bits of memory. Lastly, measurement results indicate no frequency dependent effects of radiation.
The second, third, and fourth independent studies found TegoChip technology to be fully functional even after 300+ kGy exposure. TegoChip technology demonstrates no data corruption or degradation in chip performance, unlike traditional RFID technologies that become unstable or fail after any exposure to radiation.
 9. Tekscan (Booth #316)
9. Tekscan (Booth #316)

FlexiForce force sensors can measure force between almost any two surfaces and are durable enough to stand up to most environments. The sensors are available off-the-shelf for prototyping or can be customized to meet the specific needs of your product design and application requirements.
As a result of their form factor, which is thin, flexible, and lightweight, FlexiForce sensors are ideal for OEM product integration in applications where force feedback is required. These characteristics, as well as their low power requirements, make them ideal for product designs where size and portability are important.
The sensors can be used for an array of medical applications ranging from robotic surgery and drug delivery systems to orthopedics and physical therapy devices.
10. Portescap (Booth #935)
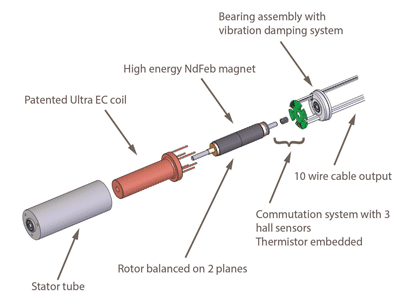 Ultra EC high performance brushless slotless miniature motors were introduced by Portescap in 2013. These miniature motors were custom developed to address the needs of medical device manufacturers that require high performance in a compact size. An example from this product line is the 22ECS, which was specifically developed for medical ventilators, and can operate at high peak current which improves motor responsiveness to ensure the best patient therapy acceptance.
Ultra EC high performance brushless slotless miniature motors were introduced by Portescap in 2013. These miniature motors were custom developed to address the needs of medical device manufacturers that require high performance in a compact size. An example from this product line is the 22ECS, which was specifically developed for medical ventilators, and can operate at high peak current which improves motor responsiveness to ensure the best patient therapy acceptance.
The high power density of this motor allows for high performance in a compact package which allows larger motors to be replaced without sacrificing performance. This reduces size and weight of end applications and increases portability. The high efficiency of this motor allows for lower power consumption and reduced energy consumption, in turn increasing battery life. These motors also run up to 30% cooler than comparative motors, thereby allowing for end user comfort, and increased life time.
About the Author(s)
You May Also Like


