May 16, 2014
|
By Rudi Gall, Raumedic Inc. |
Polymers and plastics such as thermoplastic polyurethanes, polyamides, polyolefins, thermoplastic elastomers, and plasticized polyvinyl chloride have continually made their way into medical device applications in recent decades. Increasingly, these materials have been used as substitutes for metals, glass, and other substrate materials because they offer better workability, enable greater design versatility, and lower costs. Anyone who has paid a recent visit to a hospital has witnessed the proliferation of polymers in such medical devices as tubing sets, masks, catheters, and bags. In essence, one might be inclined to say that thermoplastic polymers have been behind the great boom in generally available and low-cost medical devices.
|
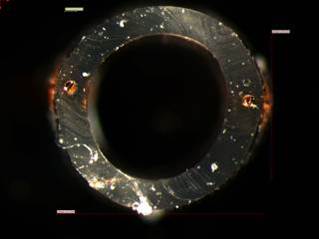
Microbore tubing features copper wires embedded in the left and right tubing walls. |
However, to be able to deliver devices with ever-increasing performance levels, medical device manufacturers continue to face many design challenges and requirements. Propelling these requirements is the unrelenting trend toward minimally invasive procedures, requiring smaller and smaller devices made from a range of microcomponents, and the trend toward increasing medical device connectivity. As a result, there is a growing need to unite plastics and such metals as stainless steel, although they used to be fierce competitors.
Both trends pose a challenge to the current manufacturing mindset focused on 'plastics only' because the material requirements of miniature devices run counter to the market demand of maintaining or improving the physical characteristics of each single plastic component. The solution to this dilemma is to be found in combining plastics with the strength and other physical properties of metals.
For example, the requirements of today's intravascular devices incorporating innovative microcatheters are beyond the physical capabilities of thermoplastic polymers. To improve the bend radius, torque response, flexibility, pushability, stiffness, and kink resistance of such devices, their inner and outer diameters must be reinforced using such structures as stainless-steel braiding or coiling.
Polymer-based medical devices requiring complex multilumen tubing must also incorporate metal structures, including guidewires, lumen-steering elements, and data-transmission wires. Such tubing can feature up to 13 lumens, which are used as fluid-transfer channels or inflation ports. In such devices, the outer diameters can be braided or coiled with metal structures to improve overall catheter performance. While wires used to be fed manually through the lumens, current technologies allow manufacturers to coextrude multiple wires across a wide variety of tubing materials to transmit data, conduct electronic impulses, and even perform radiation therapy.
|
An intracranial brain-pressure catheter from Raumedic consists of a single lumen made from an implantable-grade polyurethane catheter and wiring leading to a microchip embedded in a stainless-steel tip. |
In new neurological and neurovascular device applications, thermoplastic polymers, metals, and electronics morph into a single product. In such devices, metal cables or wires made from such materials as copper are embedded inside tubing walls or are inserted inside the inner lumen to allow for the transmission of electronic data between the device and the patient. One such device is an intracranial brain pressure catheter. Used to measure brain pressure, oxygen levels, and temperature inside the brain of severely injured trauma patients, this device consists of a single lumen made from an implantable-grade polyurethane catheter that protects wiring leading to a microchip embedded in a stainless-steel tip at the distal end of the catheter. In this instance, the wire helps to feed back valuable data from the patient to a bedside monitor.
What all of these products have in common is that they require a high degree of customization, which leads to higher engineering, development, and tooling costs. But despite these higher costs, such highly customized catheters can meet a range of application requirements and can be directly installed into assemblies without the need for costly secondary operations and assembly work.
Because such devices feature complex designs and are used to perform invasive procedures, suppliers must do their homework to reduce variability, guarantee a robust assembly process, and ensure lot-to-lot consistency. Meeting these demands requires the employment of statistical process control data, which can be achieved through the use of such in-line measurement systems as laser microscopes and ultrasound equipment.
By having a stable quality system in place, understanding the requirements of the medical device market, and offering value-added capabilities tailored to the manufacture and assembly of devices that combine both polymers and metals, single-source providers of medical device components and systems can help shape the future of the industry.
|
Rudi Gall is managing director of Raumedic Inc. (Leesburg, VA), a single-source provider of polymer components to medical device OEMs. He joined the company in 2004 to orchestrate its entry into the U.S. market. An expert in precision extrusion and molding applications, Gall has more than 15 years of sales, marketing, management, and product development experience with polymer components and systems. After receiving an MBA, he joined Rehau in 1998, first as an international controller and then as sales director for polymer hoses and tubing in Switzerland. Reach him at [email protected].
About the Author(s)
You May Also Like