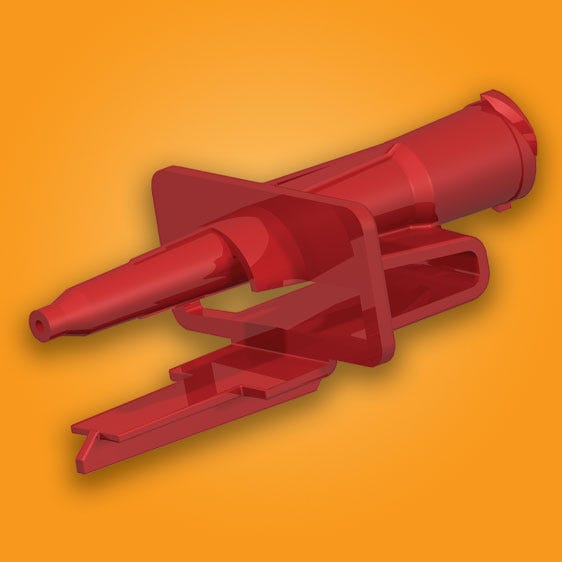
Shown here is a custom-designed taper connector set with proprietary threading to lock out several connectors, including other tapers, being employed on the same surgical set.
There are thousands of different tubing connector options on the market today that provide medical design engineers with many standard choices when developing a medical device. In some cases, however, the best option for the device design requires a tubing connector that cannot be found in the standard options offered. In such a case, the design engineer must define a special fitting and work with an experienced company to develop and produce this new part.
This article provides a detailed checklist of the areas that should be considered when specifying a custom fitting for a medical device. A checklist such as the one below can assist an engineer in making sure that the part meets the demands of the application, the cost targets defined, and the timeline required for market introduction of the medical device.
Partnering with a Connector Supplier
Working with an experienced designer that has a thorough knowledge of the fluid connector market and viable design options can make development of the fitting much easier. This is at least partly because the experienced company should also be able help an OEM determine whether the part needed already exists, even if it is manufactured by another vendor. This can spare the OEM the development time and costs to make a custom fitting.
Identify the fitting requirements and create a document to encompass these requirements. A good design and development partner will put one together, depending on the complexity of the OEM’s connector needs, to ensure that everyone is working from the same set of guidelines. This can help ensure the product’s success as well as a timely delivery within budget.
In looking for qualified partners, the OEM should seek a supplier that can demonstrate an ability to mold fittings that are similar in shape, size, and tolerance to the component needed for the design. The supplier company must also demonstrate experience in molding the plastic material required and be able to show that it can meet the OEM’s volume requirements.
Prepare the Checklist
Provided here is a checklist of items that OEMs need to consider to assist its part design and molding partner in developing the component that best meets the required specifications.
Describe What You Are Connecting
Often, if the OEM can provide a good description of the full connection, the molder can suggest a part design that may work better in the application. Keep in mind that a supplier experienced with connectors has seen a significant number of different designs and will have ideas to share on the best way to handle any challenges.
Start by identifying the details of the tubing. Define the material, size, wall thickness, and durometer of the tubing that will be connected. Depending on the application, consideration must be given to how the tubing will be attached to the fitting in the OEM’s production and how strong the attachment will be once the connected tubing and fitting are being used in the medical device. The challenge of assembling the fitting and tubing is often just as important as how the connection will function once it is in the field inside the device. If the fitting and tubing are difficult to assemble, this problem may result in additional costs or quality issues.
Explain any important features of the fitting such as threads, quick connect and disconnect, luer, sealing surfaces, etc. Give special consideration to the forces that the tubing connector might see within the medical application (e.g., side load, axial pull, pressure, etc.) and how long the connector must operate in the device. For example, connections in patient monitoring devices experience different forces in patient rooms and must work for an indefinite period of time.
Determine Whether the Part Already Exists
If the part exists, who makes it? Are the pricing, minimum order, and lead time reasonable? If the OEM works with a component design company that has experience with plastic fittings, that company may be able to offer an off-the-shelf part, or suggest a company that offers the part required.
|
This connector is specially designed to allow for precise metrics in cartridge-filling applications involving blood analysis. |
If there is no existing component that meets the specifications, is there a similar component anywhere that could be easily—and inexpensively—adapted? Many injection molders have mold bases and mold inserts that can be modified for a new component. These tools can be used if the required part is similar to another part that the molder already produces. Changing some inserts and core pins, for instance, within an existing mold can be significantly less expensive and less time consuming. Working with a company that already manufactures plastic connectors and understands critical features, as well as how best to assess dimensions and inspect the parts, is an important consideration to reduce development time and ensure a consistently functional product.
Define Requirements and Develop Concepts
The OEM should be prepared to help define the specifications of the part and provide other details, such as a concept drawing or physical example. Providing a sketch, drawing, or comparable example of what is desired is very helpful toward expediting a readily manufacturable design.
Identify the key functionality of the product so that critical dimensions and tolerances can be appropriately defined. The key dimensions and tolerances that must be held for the part are critical aspects of molding the part successfully. Be aware that specifying tight tolerances when they are not needed can drive up capital and part costs significantly. The tighter the tolerances, the more potential for additional process controls and unnecessary scrap.
If the OEM’s design includes significant complexities for molding, its development partner may suggest minor changes. Such modifications should not affect the part function, but may significantly affect the complexity of the mold, the cost of the development, and the cost of the part.
Explain the Application
Thoroughly describe what the part ultimately needs to do and how it will be used. An experienced fittings designer will have recommendations on the part configuration based on prior experience with proper fluid connection designs. The OEM’s specification should include the following:
? Flow rate requirements.
? Pressures (mininum, maximum, and working).
? Mating or connection requirements and features.
? Cycle life.
? Connect and disconnect forces.
? Any other details that might be important such as form factor, anticipated user interface, environmental factors, aesthetics, etc.
Select the Right Material
|
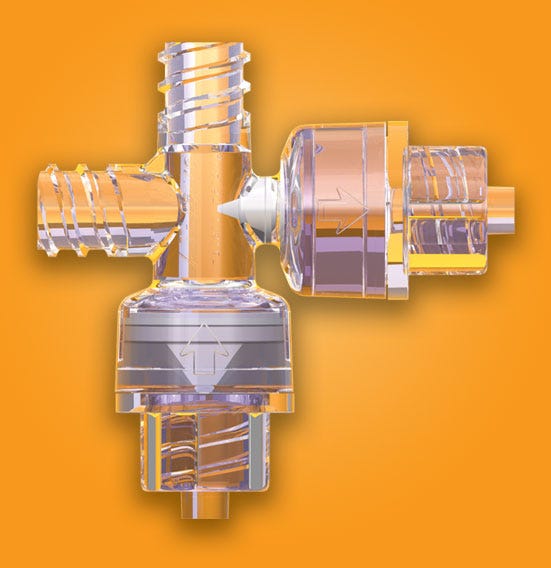
A custom four-way valve, such as the one shown here, offers efficient means of moving media in both aspiration and injection operations for surgical procedures. |
The material chosen should be based largely on the physical or mechanical requirements of the product and the type of media (and cleaning solutions) that the final medical product will be in contact with. For example, polypropylene is a good choice if a soft, molded plastic is needed while polycarbonate is a much harder plastic. Sterilization and regulatory requirements should be considered and accounted for as well. Keep your material options open as much as possible to get the most out of the design. The supplier can help identify materials that are most easily manufactured and that achieve the cost target for the component. The following material aspects are important as well:
? Mechanical properties. Traits such as toughness, ductility, lubricity, temperature capability, etc., are critical to choosing the most functional material for the application.
? Media compatibility. Choose a material that will not react to the fluids it is exposed to. A design partner should be able to advise the OEM on chemical compatibility and the most appropriate plastic resin for the application. The supplier may have leachables and extractables information to help support the decision.
? Cleaning. Is the product single use (disposable) or will cleaners such as isopropyl alcohol, bleach solutions, or ammonia potentially be used? A reusable part must withstand exposure to cleaning and still operate as if it were new.
? Sterilization. Not all plastics are suited for certain sterilization methods, so it is important to identify what sterilization methods and parameters may apply, if any. For instance, acetal’s tensile strength diminishes considerably following gamma sterilization. The material supplier should identify what sterilization methods are acceptable for each specific resin.
? Biocompatibility and regulatory requirements. Depending on the application, there may be ISO 10993, RoHS, or other requirements necessary to bring the finished device to market.
Define Cost Targets
Most applications have a defined cost target for the overall assembly, which includes a corresponding cost target for components. Work with the developer to define a reasonable cost target based on the design, material, and estimated annual usage. Volume expectations (estimated usage for at least three years) are also a critical factor in determining the cost of a component. The supplier uses the OEM’s volume estimates when determining the mold design and number of mold cavities. In addition, manufacturers should have an idea about the expected quantities per release (lot size); this is typically the volume that the manufacturer will run per lot for the component. If a molder has experience with the type of component and materials required, it should also be able to provide the manufacturer with a thorough cost estimate for the part.
There are also development costs and finance options to consider. If an OEM application involves the use of injection-molded parts, some of the following elements are involved: part design, mold design, mold fabrication, part mold and evaluation, mold adjustments and validation, and a first-article inspection process for the part. Many companies choose to cover these costs as they are incurred, e.g., amortize the cost of development over a number of parts to be molded or via other prior agreed upon arrangements.
Ultimately, the partner should be able to help the OEM balance the cost of the development of the mold with the cost of the component. High-cavity molds generally yield the lowest cost fittings, but they are the most expensive to develop. For OEMs that do not need high-volume quantities, the cost of the part may be higher.
Understand the Development Process
The development process and schedule depends almost entirely on the complexity of the design. Consider the following aspects:
? Part design and approval. Most experienced companies should be able to provide a manufacturer with a detailed part design in a CAD-based drawing for review and approval prior to the development of the tooling. This is a critical step so that all parties can agree on and understand all of the critical dimensions of the fitting.
? Tool design and fabrication. As stated earlier, some molders can use existing molds. In most cases, the tool design is incrementally more complex than the part design. The quality of the part depends heavily on how well the tool is designed and built. Partnering with a reputable and proven manufacturer is key to ensuring that both the tooling and molded parts will be of the highest quality.
? Part molding, testing, tweaking, validation, and first-article approval. It is critical that this be a relatively transparent process that allows the manufacturer and supplier to work together to ensure the parts are produced to specification and optimally functional. Such collaboration between the OEM and the supplier can result in meeting or exceeding delivery and cost expectations.
Conclusion
Tubing connectors are often some of the last parts considered in the development of a new product assembly. Correctly defining all of the details of the custom fitting and choosing a company with appropriate experience in developing and producing the connector can save significant time and money. Using the supplier considerations highlighted in this article can help manufacturers employ an outside partner in an efficient, cost-effective way.
Chuck Philipp is vice president of business development at Value Plastics Inc. (Fort Collins, CO), and Riley Phipps is technical and design services manager at the company.
About the Author(s)
You May Also Like