OEMs and suppliers respond to increasing numbers of hospital-associated infections by incorporating antimicrobial materials
April 16, 2008
For decades, the healthcare industry has tried to stay a step or two ahead of antibiotic-resistant bacteria by monitoring hospital environments and the usage of medical devices. In recent years, however, bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), also known as the Superbug, have demonstrated increased strength, the number of hospital-associated infections has continued to rise, and the occurrence of potentially harmful accidents like misconnecting IV components has proven that human error is not only to be expected, it is inevitable. While hospitals and healthcare providers try to curb these incidents in their own ways, OEMs are feeling more pressure to help prevent such problems from occurring at the device level.
Taking the Safety Initiative
|
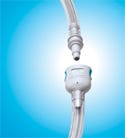
Designed to eliminate potential misconnections with luer fittings, Colder's SRC connector is made from medical-grade, animal-free polypropylene. |
The Superbug can be carried by a person, even if they aren't infected with the bacteria, or on equipment such as a catheter. It can be easily spread in a hospital where people are in proximity to each other and many patients have weakened immune systems, making them more susceptible to infection. The prevalence of the Superbug has created a demand for products that help prevent the spread of bacteria or reduce the chance of infection. "Many manufacturers are looking at adding an antimicrobial offering," says Kerry Edgar, vice president of marketing at Medegen Inc. (Ontario, CA; www.medegen.com). "With the Joint Commission [National] Patient Safety initiatives asking hospitals to reduce hospital-acquired infections. . .antimicrobial devices will begin to play a bigger role in IV products."
The Joint Commission's National Patient Safety Goals for 2008 do not instruct medical device manufacturers to use antimicrobial components; goal 7 simply states that healthcare organizations need to "reduce the risk of healthcare-associated infections." Part 7B, however, requires healthcare organizations to "manage as sentinel events all identified cases of unanticipated death or major permanent loss of function associated with a healthcare-associated infection."
MRSA enters the body through a wound or even a puncture in a patient's skin made by an IV line or catheter. By incorporating antimicrobial materials and additives in device designs, OEMs could help to eliminate their devices as potential sources of infection. Responding to the risks of hospital-associated infections, Elcam Medical Inc. (Hackensack, NJ; www.elcam-medical.com) has released an antimicrobial stopcock. Designed as an improvement on the supplier's earlier attempts to prevent contamination of IV lines, the B-Stop is made using raw material that has been impregnated with silver ions to reduce bacteria colonization on the device.
Previously the company has offered a closed, swabbable stopcock and a stopcock featuring a flow channel that automatically flushes the side port along the flow line to eliminate residual volume and to minimize potential infection. Elcam conducted in vitro tests using various yeasts and bacteria, including MRSA, to demonstrate the efficacy of the B-Stop. Results showed that the product can reduce at least 2 log of inoculated bacteria, and, in some cases, can achieve a 3-log kill, which is more than 99.9%.
Cleanliness Is Next to User-Friendliness
Though component designers and engineers can't control the environment in which their products are used, manufacturers can try to prevent a device from being misused or mishandled by creating handling instructions that support the design of the product. For example, Medegen designed its needleless luer-activated device that is used on access ports to look like a blue target.
|
The B-Stop antimicrobial stopcock from Elcam is made from raw material impregnated with silver ions to reduce bacterial colonization on the device. |
"During education of the clinical customer we remind them to look for the blue target when attaching an IV administration syringe or set," says Edgar. The blue target on the Tru-Swab port is supposed to help users remember that the port is IV related so that no other device will be connected to it. "We also remind them to follow the three Ds: disinfect it, double-check it, and dry it," says Edgar, referring to the manufacturer's instructions to disinfect the port, check it by tracing the line back to the medication to verify it is correct, and then wait for the disinfectant to dry before accessing the port to ensure maximum kill rate.
With both liability issues and regulatory factors to consider, OEMs continue to closely examine the connections they are using in IV products and how to make them safer, according to Jim Brown, medical business unit manager at Colder Products Co. (St. Paul, MN; www.colder.com). Engineers have to imagine all the potential misuses of a product at the design stage in order to make the safest possible product.
"The same concerns that are driving changes in components used in devices [in hospitals] are also affecting products used in home healthcare," says Jim Pisula, vice president of marketing for Value Plastics (Fort Collins, CO; www.valueplastics.com). "When you're designing equipment that will be used by nonprofessionals, you have to place a premium on making the designs easy to understand, friendly to patients, and durable," he adds. Brown concurs, naming misconnections as a concern for hospitals and home-healthcare applications but noting that, "ease-of-use is a bigger concern for home-healthcare applications because the user generally is less trained and [his or her] dexterity may be an issue compared with [that of a hospital professional]." Pisula also adds that more emphasis is being put on connectors earlier in the design cycle than in recent years because connectors carry significant weight in terms of customer preference and loyalty, as well as device function.
BSE, the Other Problem Pathogen
A few years ago, research showed--and FDA warned--that DEHP, a chemical used to soften PVC, could leach into the contents of an IV bag and cause harm to the patient. So OEMs began using DEHP-free materials. Similarly, connector suppliers are now noticing--and responding to--an increasing demand for and interest in animal-free materials. Bovine spongiform encephalopathy (BSE), also known as mad cow disease, is a progressive neurological disorder that can be transmitted to humans. Healthcare agencies worldwide have been recommending approaches to reducing the risk of spreading BSE to humans. One suggestion is to document that products containing animal derivatives only use materials from BSE-free countries.
Value Plastics offers its fittings and connectors in animal-free polypropylene. Because the material does not include any animal derivatives, customers are spared any BSE-related paperwork. "For years we have offered components in several animal-free materials, but the interest is greatest in animal-free polypropylene, a relatively recent addition to the medical polymer lineup," says Pisula. Brown has also noticed this trend, as well as increased interest in materials that have less potential impact on the media they handle.
Fortunately for OEMs, suppliers are equipped to offer a variety of high-performance materials to meet application and design requirements. "Device makers don't need to make a choice between patient safety and cost-effectiveness," Pisula says. "They just need to talk with their suppliers and bring them into the design process [early] to maximize their options. The result is better solutions for device makers and patient care."
Copyright ©2008 Medical Product Manufacturing News
You May Also Like