March 13, 2017
With only a small fraction of the potential patient population currently being treated, the neuromodulation market is poised for growth.
Beverly Allan and Kamran Zamanian
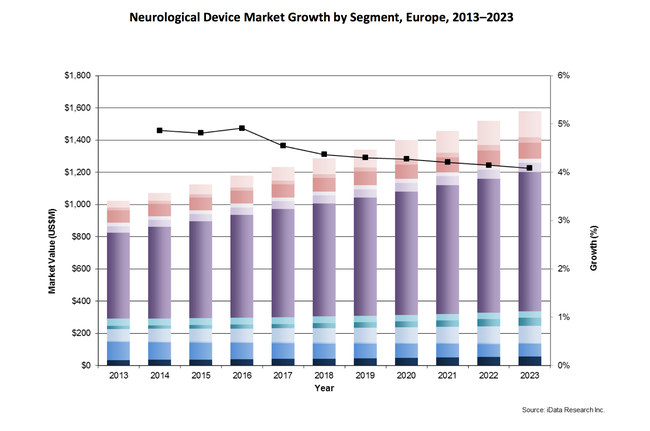
Neuromodulation is the most lucrative sector in the Europe neurological device market, accounting for over half of the revenue.1 The market is forecasted to continue growing over the next decade, as only a small fraction of the potential patient population is currently being treated with neuromoduation technology and the aging population will contribute to additional growth in the number of patients.2 Market growth will also be driven by favorable reimbursement and new products and technologies being approved across all neuromodulation sectors.
The largest sector of the neuromodulation market is spinal cord stimulators, and it will continue to produce the largest revenue over the upcoming years.1 Spinal cord stimulators deliver electrical pulses to nerve fibers in the spinal cord to mask pain signals to the brain.3 This treatment reduces chronic pain and has been used as treatment for failed back surgery syndrome, complex regional pain syndrome, neuropathic pain, and ischemia-related refractory pain. Spinal cord stimulators currently account for more than half of the neuromodulation market, and an increasing number of product approvals and positive clinical outcomes are driving growth in this market.1 Two of the leading competitors in spinal cord stimulation launched new products in 2015, with Boston Scientific releasing the Precision NoviSpinal Cord Stimulator and St. Jude Medical releasing the ProclaimElite SCS System.3,4 Both devices are nonrechargeable, and the latter also offers new burst stimulation.3,4
Nonrechargeable neuromodulation devices are significantly more common in Europe than in the United States.2 These devices tend to be less expensive, which appeals to some of the more price-sensitive countries in Europe.2 There is a larger proportion of rechargeable devices used in countries where healthcare devices tend to garner a premium price than in countries with budget constraints.2 This factor can impact the market share of leading competitors across Europe as countries differentially prioritize the cost of devices.2
This trend is also seen in deep-brain cord stimulators, which is the second largest sector of the neuromodulation market.2 These devices apply electrical stimulation to the brain and have been used as a treatment for treatment-resistant movement and affective disorders such as Parkinson's disease, tremors, dystonia, clinical depression, and chronic pain. They require an invasive procedure that can take highly skilled neurosurgeons many hours to complete. The limited number of facilities and surgeons that can perform these implants slows market growth, but as capabilities increase along with positive reimbursement trends, this market will experience high single digit growth in the upcoming years.2
The fastest growing sector of the neuromodulation market is expected to be the sacral nerve stimulator market.2 These devices have been found to be highly effective, with the majority of patients opting for replacements, which continues to drive unit sales. In addition, awareness is increasing among patients and physicians, driving new sales. Medtronic was the sole competitor in this market for many years, with its InterStim therapy system, first approved in 1994. The device treats urge incontinence, urinary retention, fecal incontinence, and urinary frequency symptoms. In 2016, a new company, Axonics, earned CE mark approval for the first rechargeable sacral nerve stimulator. It is 60% smaller than Medtronic's device.5 This new entrant has yet to launch its product on the market but is poised to have an impact on the pricing of these devices.
Other key sectors in the neuromodulation market include vagus nerve stimulators and gastric nerve stimulators. Medtronic was also the sole competitor in the gastric nerve stimulator market for many years after approval of the Enterra device in 2002. There has also been only one other company in the vagus nerve stimulator market for many years, Cyberonics, which merged with Sorin in 2015 to create LivaNova.6 These market segments are expected to experience steady growth, as new companies have launched products in both sectors that are approved for the treatment of obesity. These devices have yet to become primary treatment options, but if additional evidence of improved clinical outcomes is released, it could drive significant market growth due to the large potential patient population.
Germany currently leads the overall neuromodulation market in terms of the number of stimulators placed every year, but large markets are also present in France and the United Kingdom.1 Several countries are currently restricted to a limited number of neuromodulation implants per year, but their markets will grow as favorable reimbursement increases in the next few years, driven by physician and patient demand due to increasing evidence of positive clinical outcomes.1
Medtronic remains the global leader in the neuromodulation market, as they are the leading competitor in the spinal cord, deep-brain, sacral nerve, and gastric electric stimulator markets.1 St. Jude Medical is the second leading competitor, due to its product offerings in spinal cord and deep-brain neuromodulation.1 In April 2016, it was announced that St. Jude will be acquired by Abbott Laboratories. Other key competitors include Nevro, Boston Scientific, and LivaNova.
These companies, as well as many smaller companies that have recently entered the market, are all striving to develop stimulators that offer improved efficiency, safety, user-friendliness, and miniaturization in order to improve patients experience and clinical outcomes. As new innovative technology continues to enter the European market and awareness of the benefits of these devices increases among patients and physicians, the market will continue to grow. Neuromodulation is therefore poised to remain the leading sector of the neurological device market in Europe over the next decade as it increases in market size.
References
1. "Europe Neuromodulation, Neurovascular, Neurosurgical and Monitoring Device Market-2017." iData Research. Accessed November 25, 2016.
2. Eurostat, Statistics Explained. "Population Structure and Ageing." Accessed November 25, 2016, from http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing.
3. "Boston Scientific Launches The Precision Novi Spinal Cord Stimulator System in Europe." Press Release- June 4, 2015.
4. "St. Jude Medical Announces FDA Approval of the Proclaim Elite SCS System, a Patient-Centric, Recharge-Free, Upgradeable and MR-conditional Chronic Pain Treatment Option." Press Release- November 19, 2015.
5. "Axonics Sacral Neuromodulation System Receives CE Mark for the Treatment of Urinary and Fecal Dysfunction". Press Release- June 6, 2016.
6. BizJournals. (October 19th, 2015). "Merged Houston company begins trading under new name."
Beverly Allan is a research analyst at iData Research and was the lead researcher for the 2016 U.S Neurological Device Market Report. She is currently working on the 2016 European Neurological Device Market Report.
Kamran Zamanian, Ph.D., is president, CEO, and a founding partner of iData Research. He has spent more than 20 years working in the market research industry.
About the Author(s)
You May Also Like

