Connectors, cable assemblies, and electronic solutions are poised to power the next generation of medical devices.
The extent of medical electronic equipment used in patient care scenarios from surgical settings to imaging, diagnostics, therapeutics to patient self-care is growing exponentially. Advanced technology now being applied to new equipment and medical device development promises to bring even more innovation to benefit patient experience and outcomes.
Connectivity solutions—cables, connectors, and electronics—are embodied in many of these new designs. However, connectors don’t always get the attention they deserve. While it may sound fairly uncomplicated, connectivity is a complex endeavor. Connectors and cables are small but essential components enabling electronic equipment to function safely and properly. If a single connector fails, the whole device fails and the entire electronic ecosystem into which the device is integrated also collapses. Connectivity solutions, therefore, must deliver reliable data transmission and functionality as well as meet strict regulatory requirements for a variety of complex medical devices.
Choosing the right connectivity solutions depends upon the performance required from the medical devices to which they are applied. This article will examine some of the connectivity options and discuss which may be the most appropriate for the settings in which they will be used.
Medical Device Operational Considerations
Connectivity solutions must be safe for medical personnel and patient use, and ease of use may even be critical for safety depending upon the settings in which they will be used.
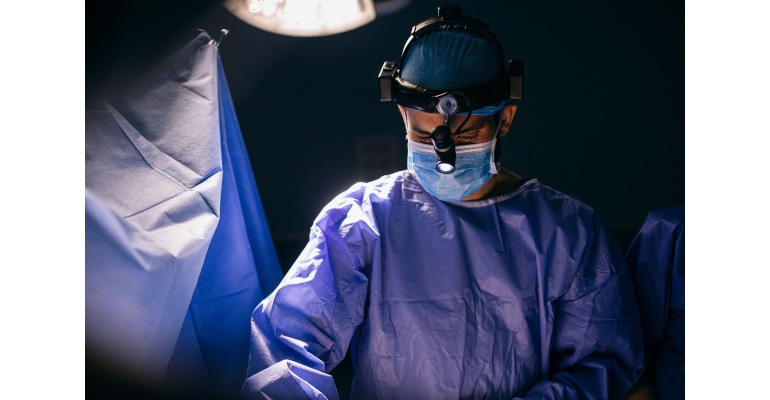
In an operating theater, for example, there can be numerous people involved in a variety of patient interventions. Surgeons may use headsets to create optimal lighting conditions during an operation. To avoid a cable slipping into the surgeon’s field of vision, a special connector solution can be deployed that is 360-degree pluggable. This ensures that the cable always stays in position, connecting straight to the device, regardless of the surgeon’s movements.
Another scenario may involve in-home patient care where wearables are becoming increasingly popular for monitoring daily regimens and delivering alerts when necessary. For this application, the right device is essential, which, through an appropriate connector solution, is lightweight and offers reliability, comfort, and usability.
The use of connectivity solutions has also become an important consideration in delivering independence for those who have suffered loss of movement. With modular and lightweight exoskeletons, the motors at the joints require transmission between the joint and the controller, which is made possible by robust connectors. As exoskeletons are mainly used outdoors to enable walking and perhaps support sports activity, these connectors must also be rugged, impervious to various weather conditions, and easy to clean. Weight is also a factor in this case. Therefore, connectors manufactured from chrome-plated aluminum are a good option for replacing traditional heavier brass connectors, which are almost twice the weight.
These three vastly different scenarios demonstrate the versatility of medical device connectivity solutions. They are available for just about any medical application that requires device/cable connectivity and operational excellence.
Determining Specifications
As medical devices are highly regulated by FDA and other agencies, connectivity solutions must meet rigid certification requirements. ISO 13485:2016, for example, is the internationally recognized quality systems standard for medical device manufacturers and also extends to their suppliers. This ensures that components are safe and reliable for integration into medical devices and that they meet quality system compliance specifications.
Environmental considerations also play a role in determining connectivity design specifications. In a medical environment with equipment for innumerable patient care situations, connectors must be resistant to dust, dirt, debris, and moisture incursion. For example, a connector with an IP68 sealing rate is protected from water ingress in both mated and unmated states. As medical equipment must be frequently sterilized, connectors and cables should also be able to withstand high temperatures associated with autoclaving and other methods of sterilization while still remaining safe and hygienic.
Other important factors include durability and ease of use. For example, connectivity solutions in a medical setting often require high numbers of mating cycles; therefore, they must be durable and sturdy enough to withstand these frequent plug/unplug situations. For example, a good measure in cases such as diagnostic and imaging solutions is at least 10,000 mating cycles. Connectivity action should be intuitive for all users—from trained to untrained— to alleviate issues with mating or damage to equipment.
Weight and size are also specifications to consider, particularly with wearables and in-home patient devices. Connectivity solutions can be customized to fit these specific configurations. With miniaturization of devices on a fast track, customized cables and connector solutions that are compact and lightweight and deliver the needed power and fast data transmission can make the difference in successful and efficient deployment.
Therefore, when medical device connectivity solutions are being designed, these specifications, among others, are important for selecting the right solution for the appropriate machine.
Choosing Locking Mechanisms
When mating connectors to the medical device, there are several locking mechanism choices to meet specific requirements to aid the coupling and uncoupling of the mating parts. These mechanisms secure the connectors in an optimal operating position and ensure the accurate electrical continuity and speed needed between the cable and the device.
Among the most robust of the options are push-pull, breakaway, and screw. Blind mating brings benefits when connectivity lacks a clear line of sight. Color-coding identification is also a common option.
Looking to the Future of Medical Devices
There is no doubt that the future will bring new ways of treating disease and managing healthcare delivery. Medical devices will continue to experience rapid technological changes that will have a dramatic impact on the practice of medicine.
According to an industry analyst report by Modor Intelligence, the global medical device market was estimated to be USD $504.85 billion in 2020 and is expected to generate a CAGR of around 5.71% over the forecast period from 2020 to reach USD $629.40 billion in the year 2026.
The wearables market is predicted to grow three-fold over a forecast period between 2018 and 2023, according to industry analyst Market Research Future. The global wearable medical device market will reach more than USD $27,200 million by 2023.
Robotics, according to Data Bridge market research, is expected to grow at a CAGR of nearly 23% during the forecast period between 2021 and 2028 and will continue to revolutionize operating theaters.
Medical instruments will, for the foreseeable future, require a vast range of connectivity solutions that will continue to evolve to meet the most challenging scenarios much as we’ve seen the innovations in hybrid options, disposables, and the introduction of new silicon and low-friction coatings. The opportunities are endless with versatile and customizable connectors, cable assemblies, and electronic solutions. With IT-enabled and interoperable medical devices, electrical and optical connectivity solutions are playing a vital role in advancing product capabilities and will continue to be a contributor to innovative advancements in healthcare.
About the Author(s)
You May Also Like




