April 1, 1998
MPMN Profile
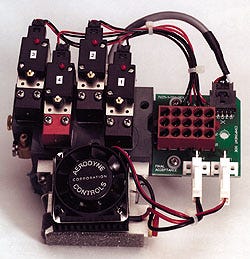
Compact Valves Help Miniaturize Device
The evolution of medical devices is toward including more functions and upgrades while reducing overall weight and size. Arrow International (Everett, MA), which has specialized in the development and manufacture of intra-aortic balloons (IAB) and intra-aortic balloon pumps (IABP) since 1969, had a successful product on the market with their KAAT II Plus IABP. Nevertheless, Arrow engineers knew that in order to stay ahead of the competition, they would have to continually improve the design of the device.
The Smaller the Better
A model of compactness, the KAAT II Plus has no exposed parts. All of its connections are recessed and are accessible from one side of the pneumatic pump. The system includes either a lightweight 500-psi helium canister that provides a minimum of 21 days of continuous pumping or a refillable 2000-psi D-size cylinder that provides an extended helium supply.
Microprocessor controls and a stepper motor/bellows drive system deliver gas shuttle speeds for one-to-one pumping, even in patients with high heart rates and tachyarrhythmias. Accuracy of the delivered volume and the ability to set volume in 0.5-cm3 increments ensure patient safety and maximum effectiveness of the IABP assist.
In addition, all operating parameters are preset on system start-up, and most pump functions are already calibrated so that the IABP starts pumping at full volume within a few seconds of turning it on. An automatic purge at start-up means counterpulsation begins in less than one minute.
Seven distinct trigger modes provide maximum flexibility and the capability to pump in any situation. Either conventional timing or real-time R-wave deflation trigger/timing meets any clinical situation. The instrument continuously monitors balloon volume, pressure, and all operating systems. Should an alarm sound, the pump indicates which alarm is involved. A step-by-step troubleshooting procedure is also displayed. If a helium leak is detected, the pump automatically shuts down and prints the last 6 seconds of wave forms.
Where to Next?
The KAAT II Plus found immediate acceptance in the industry. Nevertheless, Arrow's engineering team had their eyes on the future. The Arrow team felt that the product could be further refined--particularly the large manifold and solenoid valve system. So in early 1993, the engineering team was already thinking about redesigning the IABP.
It was then that Dick Graeb, director of sales and marketing at Aerodyne Controls Corp. (Ronkonkoma, NY), made initial contact with Arrow International. Aerodyne designs and manufactures custom pneumatic valves and pressure regulators, solenoid valves, manifolds, assemblies, centrifuges, and nonmercury motion switches. Already well represented in the military and aerospace markets, this custom manufacturer was determined to break into the medical market.
Aerodyne's first opportunity came when Arrow contracted them for the redesign of one of the accessories of the KAAT II Plus: the check valve between the IABP's small and large helium canisters. The result was an extremely light (1.2 lb) component able to handle 2000 psi. The new valve did more than meet the required specifications; it gave Arrow the confidence to work with Aerodyne on the greater project of miniaturizing the IABP's manifold/solenoid assembly.
Heading up Arrow's team of engineers was project manager Mike Jesi. Aerodyne's design team was led by president Jim Miller and vice president of engineering Len Tetrault, both of whom were involved in the daily management of the new assembly's development.
Cross-Over Experience Counts
Was Aerodyne's military background a concern? "On the contrary," says Jesi. "First of all, they had a solid understanding of pneumatic controls. Secondly, they were accustomed to the rigors of documentation, compliance, and confidentiality."
Aerodyne's Tetrault says, "Often it's our experience in the application of our products that customers find valuable. The engineers at Arrow were about to develop this assembly in-house, yet they allowed us to do it, having seen just how significantly we were able to reduce not only volume and weight, but also noise and power requirements in other applications.
"A further consideration," Tetrault continues, "is concurrent engineering--in which design, manufacturing, assembly, and quality assurance specialists provide input from the beginning of the project. This process allows us, in almost every case, to increase production efficiency."
After approximately one year the Arrow team came to Ronkonkoma to witness the testing, which was a fairly complicated process. The test setup used an actual KAAT II Plus to ensure the assembly performed to specs. Not only did the assembly have to perform all mechanical and electrical functions properly, but it also had to respond correctly to the extremely sophisticated software commands on which the timing and safety features of the apparatus depends. Fortunately, the Arrow team was very pleased, and no significant modifications were required.
And the Beat Goes On
The result of this collaboration is a new IABP manifold/solenoid assembly that sits comfortably in the palm of the hand. Beyond miniaturization, one of the major benefits to the new assembly was enhanced reliability. Much of the plumbing (the pipes and fittings) was eliminated by manifolding, which in turn resulted in the elimination of potential leak points. A further result, and perhaps the most important, was reduced life cycle costs. The commitment to redesigning the pneumatics did represent an investment, but after virtually eliminating repair costs, service calls, and attendant downtime, the return on investment has been exceptionally fast and has reduced the number on the bottom line.
Arrow's new IABP is currently in the 510(k) process, and its introduction to the medical market is anticipated soon. With evolution in medical instrumentation having been served, a more compact and reliable IABP will soon be available.
You May Also Like


