The Flagstaff, AZ-based company brings the Cardioform ASD Occluder to Europe a few months after the device received approval in the U.S.
October 2, 2019
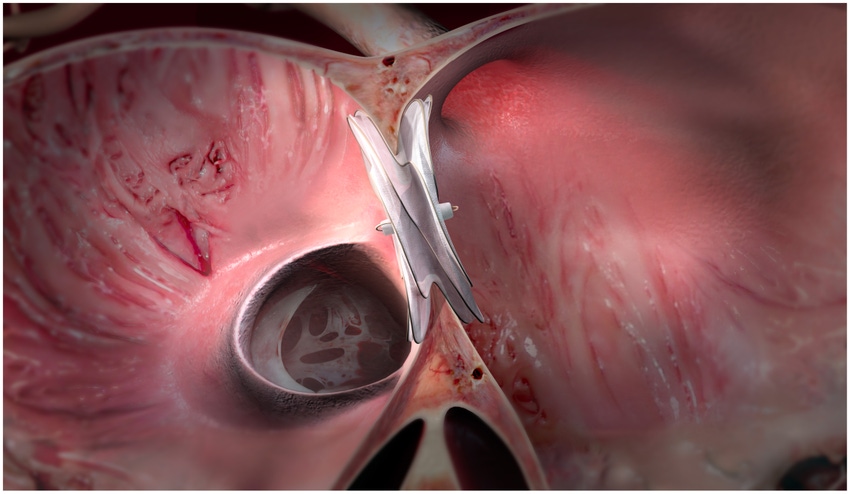
W.L. Gore is bringing its Cardioform ASD Occluder to Europe. The Flagstaff, AZ-based company announced Wednesday it received CE mark for the device, which can close atrial defects (ASDs).
The device expands the size of what previous devices in the Cardioform line can treat said, Jake Goble, MBA, PhD, leader of Gore’s Structural Heart Pipeline.
“Our devices in the past have only treated up to 17mm defects and that represents maybe about half of all defects that are out there,” Goble told MD+DI. “This recent approval allows us to be able to treat larger defects. This new device extends that treatment range up to 35 mm in diameter.”
Gore’s Cardioform ASD Occluder met its primary endpoint for the percutaneous, transcatheter closure of ostium secundum atrial defects (ASDs) in a clinical trial. The Gore ASSURED Clinical Study demonstrated safety, closure, and technical success that statistically achieved the primary endpoint.
The pivotal study evaluated the safety and efficacy of ASD closure using the GORE CARDIOFORM ASD Occluder in 125 patients with evidence of right heart volume overload demonstrating the need for defect closure. The study involved patients between the ages of two and 84, across 22 investigation sites, including 15 children's hospitals.
CE mark for the Cardioform comes just a few months after Gore scored a nod from FDA for the product. Gore launched the device in the U.S. in August. Plans now turn toward rolling out Cardioform in Europe.
“Anytime we roll out technology there are a variety of things that we want to consider,” Goble said. “We want to make sure that first of all, we’re setting up the launch to have excellent patient outcomes. With the launch of the ASD Occluder in Europe, one of the things that will help shape that launch plan is how we can effectively train physicians on the appropriate use of the product.”
He added, “we have a lot of implants in our U.S. clinical trial that gives us a good basis to inform a training program and we will be in the process in the next several months of rolling out the technology country-by-country to key institutions that have seen many of these patients that can benefit from it, and we’ll expand from there.”
About the Author(s)
You May Also Like




