The Alameda, CA-based company said CHEETAH is a post-market study for the Indigo System with CAT RX Aspiration Catheter.
October 11, 2019
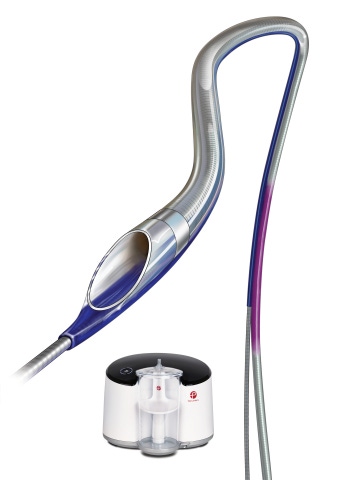
The first patient has been enrolled in Penumbra’s CHEETAH post-market study to evaluate the Indigo System with CAT RX Aspiration Catheter in the coronary vessels. About 400 patients are expected to be enrolled at 25 centers in CHEETAH.
Alameda, CA-based Penumbra’s Indigo System uses mechanical power aspiration to remove thrombus in the coronaries.
The primary study endpoint is a composition of cardiovascular (CV) death, recurrent myocardial infarction (MI), cardiogenic shock or new or worsening New York Heart Association (NYHA) Class IV heart failure within 30 days. Secondary endpoints include final TIMI flow grade, final TIMI thrombus grade, and safety assessments at six months.
“Penumbra has adapted over 10 years of neuro thrombectomy experience to address the limitations of traditional manual aspiration for the coronaries by development of the Indigo System CAT RX device,” said national principal investigator S. Jay Mathews, M.D., director, cardiac catheterization laboratory, Manatee Memorial Hospital in Bradenton, Florida. “This significant upgrade in innovation from syringe-based aspiration to mechanical power aspiration coupled with highly trackable catheter technology has enabled us to improve our door to reperfusion times and thereby to improve patient care.”
In 2014, Penumbra introduced the Indigo System, a continuous aspiration mechanical thrombectomy system designed to remove clot from arteries and veins in the peripheral vasculature. The Indigo System CAT RX Aspiration Catheter was introduced in 2018.
During an earnings call held in August, Adam Elsesser Penumbra’s chairman and CEO spoke about the potential impact of Indigo.
“When we first launched our Indigo System four years ago, we estimated that 150,000 patients could benefit from our system,” Elsesser said according to a transcript of an earnings call from Seeking Alpha. “Now four years later, with a greater understanding of the variety of cases and with advancements in training and techniques, we believe the Indigo System has the potential to address over 350,000 patients per year in the US.”
About the Author(s)
You May Also Like




