The latest generation of Medtronic's self-expanding transcatheter aortic valve replacement (TAVR) system, the Evolut FX, is designed to enhance ease-of-use and predictable valve deployment.
August 24, 2021
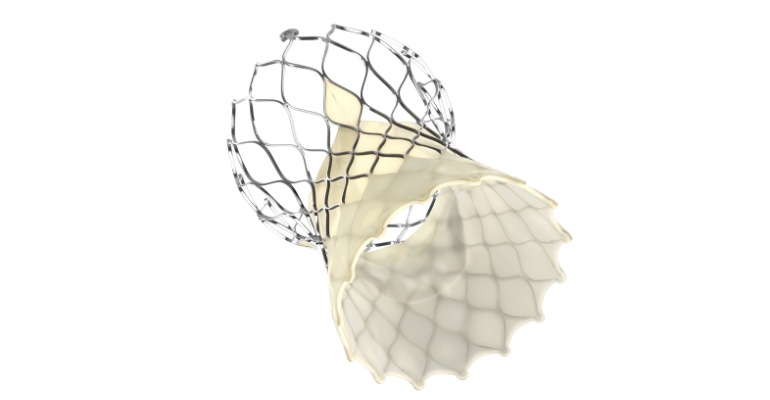
Touting improved ease-of-use and predictable valve deployment, Medtronic revealed Tuesday that FDA has approved the latest generation of the company's self-expanding transcatheter aortic valve replacement (TAVR) technology.
"FX is going to be a really big improvement and the predictability of [the vavle deployment] has been really a home run for us," Sean Salmon, president of Medtronic's diabetes unit, and president of the cardiovascular portfolio, said Tuesday during the company's fiscal first quarter 2022 earnings call.
Medtronic CEO Geoff Martha said the company plans to start rolling out the new system in the U.S. market later this fall. The company noted that a full launch is planned for early in 2022.
"Now this innovative system is designed to improve the overall procedural experience through enhancements and deliverability, implant visibility, and deployment stability," Martha said during the earnings call Tuesday.
Medtronic noted that the Evolut FX TAVR system incorporates the same supra-annular valve design that has shown hemodynamic performance superior to surgical aortic valve replacement across large-scale, randomized clinical trials. The fourth-generation Evolut technology is equipped with gold markers built into the frame to provide implanters with direct visualization of depth and valve leaflet location during implant. In addition, the Evolut FX system incorporates a redesigned catheter tip for a smoother insertion profile, a more flexible delivery system that allows for 360-degree freedom of motion, with a stable, predictable deployment.
The newest system includes four valve sizes for the largest indicated patient treatment range and the lowest delivery profile currently on the market, Medtronic said.
“The self-expanding, supra-annular Evolut platform has evolved considerably over time and has brought heart teams innovative features like recapturability, an expanded size matrix, and advanced valve sealing to help minimize paravalvular leak. Today, the Evolut FX system further refines a trusted platform with key product and procedural enhancements that make the self-expanding system easier to use with enhanced visualization capabilities for orientation and depth,” said Jeffrey Popma, MD, vice president and chief medical officer for the coronary and renal denervation business and the structural heart and aortic business, which are part of Medtronic's cardiovascular portfolio at Medtronic.
Severe aortic stenosis occurs when the aortic valve leaflets become stiff and thickened and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body. Severe aortic stenosis often reduces a patient’s quality of life and limits their daily activities. If left untreated, patients with symptomatic severe aortic stenosis can die from heart failure in as little as two years.
The Evolut TAVR platform is indicated for symptomatic severe aortic stenosis patients across all risk categories (extreme, high, intermediate and low) in the U.S.
About the Author(s)
You May Also Like


