August 1, 2017
The company launched its CoreValve Evolut Pro device in Europe for patients in the intermediate, high, and extreme risk category for open surgery.
Amanda Pedersen
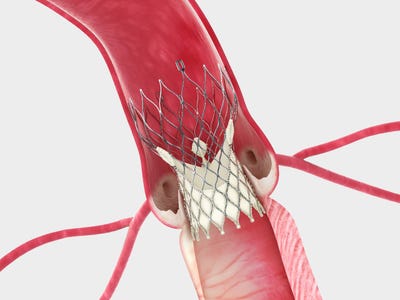
Transcatheter aortic valve replacement (TAVR) technology has evolved by leaps and bounds since the first generation of the devices were brought to market. TAVR players are not only working on new and improved versions of the technology but are also pursuing expanded indications to make the procedure available to as many patients as possible.
Medtronic is the latest company to score a new TAVR victory, this time in Europe. The company said it received CE mark for the European launch of its CoreValve Evolut Pro device for patients who fall in the intermediate, high, or extreme risk category.
The Evolut Pro device is designed with an outer wrap that adds surface area contact between the valve and the native aortic annulus, which is intended to further advance valve sealing performance. The idea is that the porcine pericardial tissue wrap will help prevent blood from leaking through the sides of the valve.
CE mark clearance was supported by a 60-patient clinical trial of the device. Data from that study shows the Evolut Pro is associated with a high rate of survival (98.3%) and low rate of disabling stroke (1.7%). The valve also shows strong hemodynamic performance, the company said, and 72.4% of patients experience minimal paravalvular leak (PVL). Also, only 10% of patients needed a pacemaker implanted after having the TAVR procedure. The rate of patients who end up needing a pacemaker shortly after TAVR has emerged as a common frustration for physicians who perform TAVR.
The Evolut Pro is delivered through Medtronic's EnVeo R delivery catheter system and is indicated for vessels down to 5.5 mm.
Earlier this year Medtronic reported two-year data from its Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial. That global study, which compared the CoreValve and CoreValve Evolut R systems to open-heart surgery, enrolled 863 TAVR patients to 794 surgical replacement patients. These were the five key takeaways from the data.
The global transcatheter aortic valve replacement (TAVR) market grew about 17% during the second quarter, compared to the first quarter, according to a recent report from Needham & Co.'s Mike Matson.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
[Image credit: Medtronic plc]
About the Author(s)
You May Also Like




