The TriClip transcatheter edge-to-edge repair system demonstrated significant reduction in tricuspid regurgitation in late-breaking data.
May 18, 2023
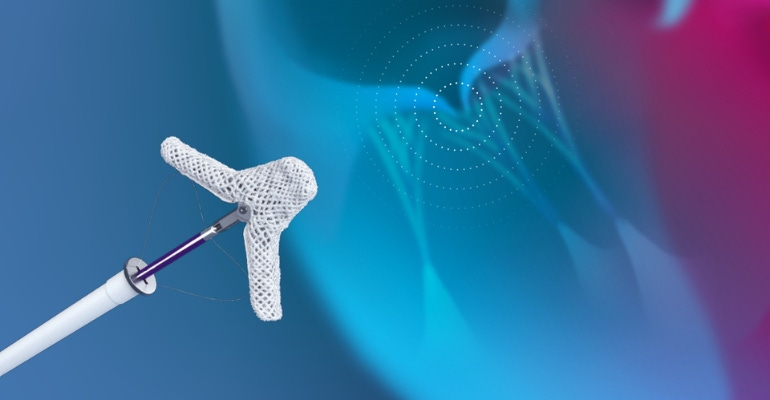
Late-breaking data presented at EuroPCR in Paris, France this week supports the benefits of Abbott's TriClip transcatheter edge-to-edge repair (TEER) system in treating patients with leaky tricuspid valves. The Abbott Park, IL-based company said the TriClip device is a first-of-its kind, minimally invasive device designed specifically for tricuspid heart valve repair.
The results of the bRIGHT study, the largest real-world dataset for transcatheter tricuspid valve repair, support the safety and effectiveness of the TriClip system in patients with tricuspid regurgitation (TR). Outcomes from 511 patients in 26 sites across Europe for this prospective, multi-center, single-arm post-market study were presented for the first time at EuroPCR 2023.
Key findings of bRIGHT study through 30 days include
Significant TR reduction. Repair with the TriClip system resulted in reduction of TR grade to moderate or less for 77% of the patients.
Significant clinical and quality of life improvements. 79% of participants achieved New York Heart Association (NYHA) Functional Class I/II (meaning they reached a point of slight or no limitation of physical activity), a nearly 60% improvement from the baseline proportion of 20%. In addition, more than half (56%) of patients reported a 15-point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score (a self-assessment of symptoms, physical and social limitations, and quality of life), representing a substantial improvement in quality of life and health status.
A strong safety profile. Only 2.5% of patients who received the device experienced a major adverse event, a composite of cardiovascular death, heart attack, stroke, new onset of kidney failure, and surgery for device-related adverse events.

"Many patients currently undergoing tricuspid TEER are at an advanced stage of the TR disease and experience severe symptoms, impacting their overall health and well-being," said Philipp Lurz, MD, PhD, professor and deputy head of cardiology at the Heart Center Leipzig at University of Leipzig, in Leipzig, Germany. "Building strong clinical evidence around the value of a procedure like tricuspid valve repair is critical, and the real-world outcomes of the bRIGHT study reinforce the safety and effectiveness of TriClip in reducing TR and improving quality of life."
TriClip is approved for use in more than 50 countries, including in Europe and Canada, and is an investigational device in the United States.
About the Author(s)
You May Also Like




