VenoValve has the potential to offer relief for patients that suffer from severe chronic venous insufficiency (CVI) in the deep veins of their legs.
August 5, 2021
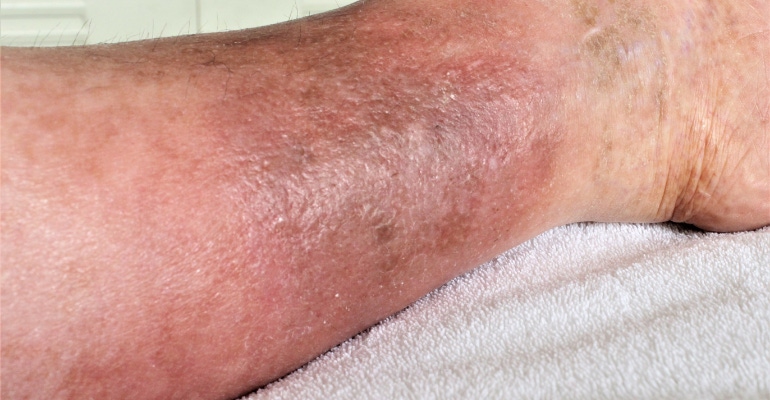
FDA continues to make use of its breakthrough device designation program, which was created in response to the 21st Century Cures Act and replaces the agency's previous expedited access pathway and priority review for medical devices. This week, the agency granted breakthrough device designation status to Hancock Jaffe Laboratories' VenoValve for the potential treatment of severe chronic venous insufficiency (CVI).
The Irvine, CA-based company has completed a first-in-human study of the VenoValve, and is finalizing plans for a U.S. pivotal trial, SAVVE (Surgical Anti-reflux Venous Valve Endoprosthesis).
Hancock Jaffee CEO Robert Berman answered the following questions for MD&DI about the VenoValve, CVI, and the upcoming trial.
What is the clinical need that VenoValve solves?
The VenoValve addresses chronic venous insufficiency, or CVI, a disease that occurs when the valves inside of the veins of the leg fail, causing blood to flow in the wrong direction and pool in the lower legs. The pooling blood causes increased pressure inside of the veins of the leg. Typical symptoms of severe CVI include leg swelling, pain, and venous ulcers that are very difficult to heal. The vascular community has been trying to solve deep venous CVI for decades, with little success. There are currently no effective treatments for the 2.4 million people in the U.S. that suffer from severe deep venous CVI, with compression stockings being the current standard of care. We are hopeful that the VenoValve will result in a paradigm shift in the treatment of CVI, and that it will be the first of several successful products that we develop for the treatment of venous disease.
How does the VenoValve work?
The VenoValve is made using a non-coronary leaflet from a pig’s heart valve, and is designed to open and close under the unique pressure and flow gradients of the venous system, which is very different than the coronary system. For example, a heart valve may open and close 80 to 120 times a minute, while a venous valve is likely to open and close 9 to 20 times a minute depending on what the patient is doing. It is challenging to design a valve that can function within the unique hemodynamic environment of the venous system and will not cause thromboses. We are optimistic that we have come up with the right combination of elements for the valve and the right implantation procedure to finally bring relief to the millions of patients that are suffering from this debilitating disease.
What data supports the use of VenoValve for CVI treatment?
To begin with, we have many years of experience working with bio-prosthetics. We have assembled a world-class team with expertise in venous disease and the hemodynamics properties of the venous system. In addition, because of the unique physiology of the human leg, there was no good animal model to test the VenoValve. There is no animal that walks upright on two legs and has the same calf muscle function and flow dynamics as the human leg. So after consultation with the FDA, we agreed to conduct a small first-in-human trial. That trial, which was in essence a dress rehearsal for the pivotal trial, was very successful, resulting in decreases in the backwards flow of blood (54%), and significant improvements in disease manifestations (56%) and pain (74%). In addition, we experienced remarkable venous ulcer healing, with no ulcer recurrences. The protocol for our pivotal trial is very similar to what we used for the first-in-human trial and we are hopeful that we will have similar results.
How will breakthrough device status help VenoValve?
We approached the FDA early on in the development of the VenoValve and have developed a nice working relationship with them. Any time a company comes up with a first-in-class product for a disease where there are no pre-existing devices, there is a learning curve for everybody involved, and we are very appreciative that the FDA has worked with us to understand the unique qualities of the venous system and our device. Breakthrough status does a few things for us: number one, it is nice to see the FDA acknowledge the debilitating impact of CVI and the potential of our product in the treatment of the disease. It also sets up our device for priority treatment from the FDA, although we have been very pleased with the responsiveness of the FDA up until now. In addition, breakthrough device status could have certain advantages as we seek to establish reimbursement and insurance coverage for our product. The bottom line is that there are millions of patients suffering from debilitating, deep venous CVI with no current effective treatment options and any help that we can get to bring relief to these patients sooner, such as breakthrough device designation, is a good thing.
What are your plans for the pivotal trial?
The SAVVE pivotal trial will consist of 75 patients at up to 20 sites. The protocol is very similar to what we used for our very successful first-in-human trial. We have had no problem recruiting sites, which consist of some of the most well-respected hospitals throughout the country and several key opinion leaders in the practice of vascular medicine. They are as excited as we are about the potential for an effective treatment for a disease that has frustrated the vascular community for decades. We expect to begin the trial in the next 60 days so anyone that wants more information about the SAVVE study, including patients suffering from CVI that might want to participate in the trial, should visit www.VenoValve.com to learn which trial site is closest to them.
About the Author(s)
You May Also Like


