Medtronic has now faced three recalls related to its HeartWare HVAD System since pulling the device from the market last year.
August 29, 2022

A year after pulling its HeartWare Ventricular Assist Device (HVAD) System from the market, Medtronic is still dealing with regulatory issues associated with the device.
In a recent update, FDA called attention to a recall Medtronic initiated in late June related to the increase in battery electrical faults due to an interaction between the HeartWare battery software and an internal component. When this happens, batteries with an electrical fault may be unable to power the controller, unable to accept charge from the battery charger, or appear to remain charged when in use. Medtronic reports 1,159 complaints, six injuries, and one death related to this issue.
This marks the third HeartWare recall since the device was discontinued on June 3, 2021.

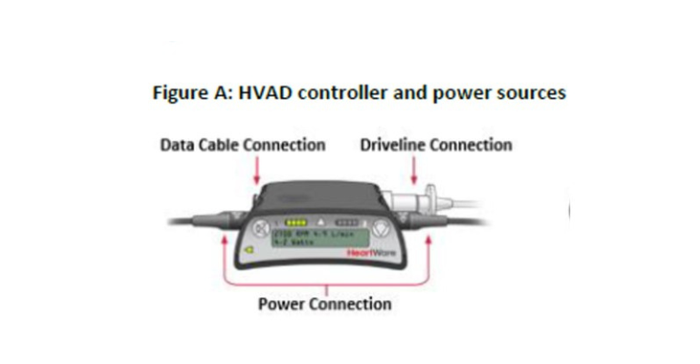
According to FDA, Medtronic told healthcare providers it will begin exchanging HVAD power sources (batteries, AC and DC adapters) and Monitor data cables with newly designed components. The company has also updated the device labeling with information regarding useful life and inspection of HVAD System components.
The HeartWare System is designed to help the heart continue to pump blood to the rest of the body. The device is used as a bridge to transplant for patients who are at risk of death from end-stage left ventricular heart failure, for heart tissue recovery, or as destination therapy in patients for whom heart transplants are not planned. The HVAD System operates using power from either AC or DC electricity or batteries.
Medtronic pumped out $1.1 billion to acquire HeartWare in 2016. It was the company's largest deal since the 2015 Covidien acquisition. However, after a growing body of observational clinical comparisons indicated a higher frequency of neurological adverse events, including stroke, and mortality with the Heartware System compared to other circulatory support devices available to patients, Medtronic discontinued the device last year. The HVAD System brought in $141 million in Medtronic's fiscal year 2021.
Even at the time of the acquisition, HeartWare was having issues with MVAD, its next-generation device, and had halted a trial for CE mark citing an increased potential for blood clots in the pump. But Medtronic was eager to buy HeartWare because at the time, St. Jude Medical had acquired HeartWare's rival, Thoratec. Abbott later acquired St. Jude and inherited Thoratec's device.
What to do if you have a HeartWare device
For patients currently implanted with a HeartWare System, Medtronic asked customers to take the following actions to help address the battery electrical fault.
Keep two sources of power connected to their controller and have fully charged spare batteries available at all times.
Acknowledge and report alarms.
Follow the instructions for use for proper power source management.
Be vigilant if the battery indicator lights do not decrease over time while the battery is in use.
If the lights do not decrease over time while the battery is in use, this could be a sign of battery electrical fault and the battery should not be used.
Pay attention to the battery indicator lights.
Do not use a battery if the indicator lights do not light up.
Check to make sure:
Battery capacity display lights up
Battery indicator on the controller lights up
Battery charger status light does not flash red or yellow after connecting the battery
About the Author(s)
You May Also Like




