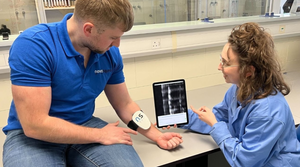
Photo of Reprieve Medical team with the company's diuretic and fluid management system for acute decompensated heart failure.
Startups
New Cardiovascular Company Emerges from Stealth ModeNew Cardiovascular Company Emerges from Stealth Mode
Fresh off a $42 million series A financing, Reprieve Medical is developing an intelligent automated fluid management system for heart failure patients.
Sign up for the QMED & MD+DI Daily newsletter.
















.png?width=300&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)