May 15, 2017
Cook Medical's Flourish device uses magnets to pull the upper and lower esophagus together, allowing food to reach the stomach.
Nancy Crotti
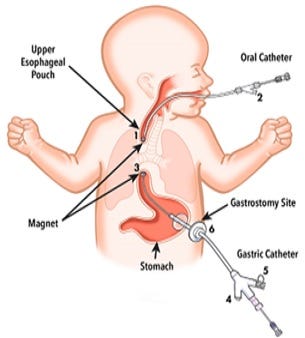
Cook Medical's Flourish pediatric esophageal atresia device is used to non-surgically repair the esophagus in infants who were born with their upper esophagus disconnected from their lower esophagus and stomach.
FDA has given a non-surgical device to help infants feed normally a humanitarian use approval.
Cook Medical's Flourish pediatric esophageal atresia device is designed to treat infants born with a gap in their esophagus. The condition, known as esophageal atresia, affects about one in every 2,500 babies born in the United States, according to FDA.
Infants with this condition cannot feed normally, and they require a feeding tube until surgery can be performed to attach the esophagus to the stomach. Most babies born with esophageal atresia also have a tracheoesophageal fistula, which also needs to be repaired surgically, since fluids from the esophagus can get into the airways and interfere with breathing.
The Flourish device uses magnets to pull the upper and lower esophagus together, closing the gap and allowing food to enter the stomach. To insert the device, doctors insert two catheters, one through the mouth and one through the stomach. The magnetic ends of the two catheters attract each other, and this attraction pulls the two ends of the esophagus together over several days. The surrounding tissue grows together, and within 13 days, the tissue trapped between the magnets will die and a hole (anastomosis) will open between the two portions of the esophagus.
Once the catheters are removed, the infant can begin to feed by mouth, according to FDA.
The agency reviewed data for the Flourish device through the humanitarian device exemption (HDE) process. An HDE is similar in both form and content to a premarket approval (PMA) application, but is exempt from the effectiveness requirements of a PMA, FDA spokeswoman Deborah Kotz said.
The agency does not require an HDE applicants to provide the results of scientifically valid clinical investigations demonstrating the device's effectiveness. Applicants must, however, provide enough information for the agency to determine whether the device poses an unreasonable or significant risk of illness or injury, and that its health benefits would probably outweigh its risk of harming patients.
Applicants must also demonstrate that no comparable devices are available to treat or diagnose the disease or condition, and that they could not otherwise bring the device to market.
Of the 16 infants who received a Flourish device, 13 developed a complication that caused a narrowing in their esophagus that required a balloon dilation procedure, a stent, or both to repair. Anastomotic strictures also occur from traditional surgery to repair the condition, FDA noted.
When asked if parents were told that follow-up procedures were a possibility before the Flourish device was used in their child, Marsha Lovejoy, a Cook Medical spokeswoman, told Qmed that the U.S. infants who received the device prior to this approval received it under emergency use, and that the physicians would have notified parents of the potential risks.
The device is not cleared outside the United States, Lovejoy said.
Kotz told Qmed the agency plans to post a summary of safety and probable benefit (SSPB) document on FDA's website within the next few weeks, which will explain more about the clinical data.
The Flourish device should not be used in patients older than one year, or who have teeth, which may damage the oral catheter. The device is also contraindicated in infants who have an existing tracheoesophageal fistula or who have esophageal segments that are more than four centimeters apart.
Potential complications include ulceration or tissue irritation around the catheter implanted in the stomach, and gum irritation due to pressure from the oral catheter. Potential long-term complications include gastroesophageal reflux.
Nancy Crotti is a contributor to Qmed.
[Image credit: FDA]
About the Author(s)
You May Also Like


