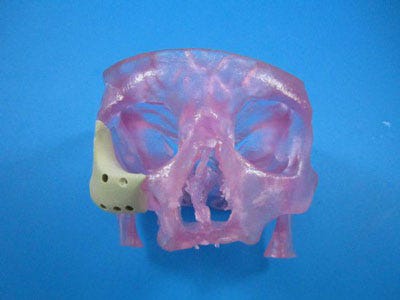
FDA doesn’t see the need to pile on new regulations to address 3-D printing.
December 21, 2015
1 Min Read
|
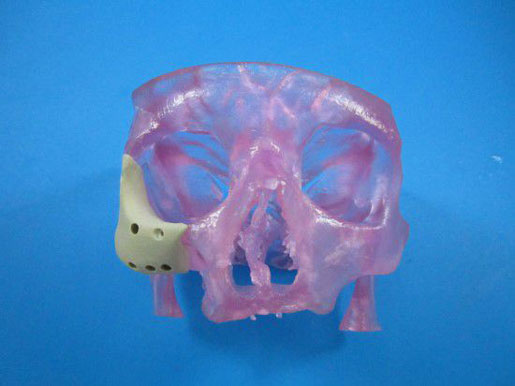
A CDRH representative said the agency views additive manufacturing as an enabling technology, just like CNC machining. |
|
|
[Image courtesy of OXFORD PERFORMANCE MATERIALS] |
Check out the future of medical technology at the world's largest medical design and manufacturing event—register for the MD&M West Conference, February 9-11, 2016. |
Sign up for the QMED & MD+DI Daily newsletter.
You May Also Like