Sign up for the QMED & MD+DI Daily newsletter.
Chris Newmarker
August 25, 2016
1 Min Read
No. 83 Edwards Lifesciences
Innovation Premium: 41.3%
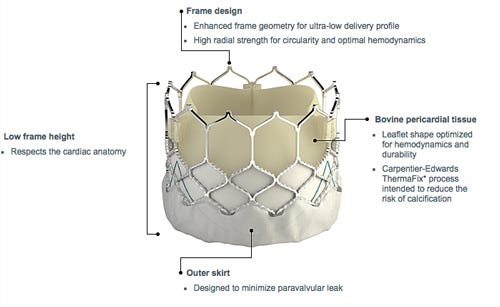
Edwards Lifesciences continues to make great strides in the lucrative transcatheter aortic valve market. Just this month, Edwards's Sapien 3 valve became the first TAVR valve to receive an expanded FDA indication that included patients with intermediate risk of dying or suffering serious complications during open-heart surgery, versus the previous indication for only high risk patients.
10 Medtech Startups on Fire in Q2 2016>>
About the Author(s)
You May Also Like