May 13, 2014
|
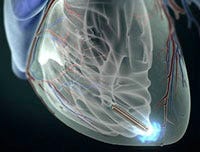
The Nanostim leadless pacemaker is designed to be implanted directly into the heart. (Courtesy St. Jude Medical Inc.) |
St. Jude Medical (SJM), which began its Nanostim Leadless Pacemaker Observational Study in March, had suspended enrollments in the trial in April but now intends to resume them soon, according to MassDevice.com.
SJM had called the halt when researchers reported six instances of perforation, including two patient deaths, among the first 200-plus recipients, reports MassDevice's Arezu Sarvestani, quoting a note from Leerink Swann analyst Danielle Antalffy.
Sarvestani says the study had been suspended in April in response to the patient deaths. "The company evaluated the reports and presented its findings to the trial's steering committee, which unanimously agreed to resume enrollment," she reports.
"St. Jude's analysis determined that the adverse events were due in part to inappropriate patient selection and in part to operator inexperience, according to Antalffy's note," Sarvestani continues. She further says that Antalffy wrote, "The company determined that five of the six perforations 'would not have occurred if the European registry aligned with the US pivotal [trial] inclusion/exclusion criteria.'"
SJM is "updating the study protocol to match US enrollment criteria, and the company is making efforts to ensure all investigators are updated and on board with the new guidelines," according to Sarvestani.
Antalffy also says that the adverse events haven't dampened physicians' enthusiasm for Nanostim. In her note, as reported by Sarvestani, she wrote, "Enthusiasm for leadless pacing ... was unanimous among all physicians with whom we spoke. One physician expects to use leadless pacing in 100 percent of his single chamber patients, while others pegged the market at 10-20 percent of patients receiving pacemakers - potentially positioning both STJ and MDT for meaningful market share gains upon approval."
Earlier this month at Heart Rhythm 2014, the Heart Rhythm Society's 35th Annual Scientific Sessions, lead author Vivek Reddy, MD, presented the first one-year results of the European Leadless clinical trial of the Nanostim pacemaker. Reddy reported no instances of infection, no failure to pace, no mechanical failures or early battery depletion, and no device migration among the 32 early implantees.
The Nanostim device trials are being closely watched because the next-generation pacemaker has no trouble-prone leads and is about one-tenth the size of conventional pacemakers. SJM bought the Nanostim company late last year, just after the device received CE Mark approval.
Doubtless SJM is well aware that Medtronic is hot on its heels with its own tiny leadless pacemaker, the Micra Transcatheter Pacing System. Medtronic is also involved in pivotal trials for its own device.
Stephen Levy is a contributor to Qmed and MPMN.
About the Author(s)
You May Also Like