October 17, 2016
A new FDA approval allows patients with a wide range of Medtronic cardiac rhythm and heart failure devices to undergo MRI scans anywhere in their body.
Joyce Laird
|
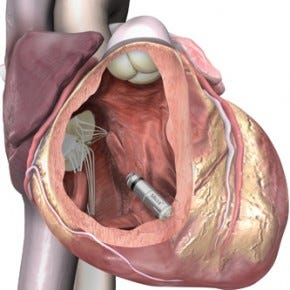
Medtronic's leadless, transcatheter Micra pacemaker is among those covered by the approval. (Image courtesy of Medtronic) |
Medtronic received FDA approval for MRI compatibility for their full suite of cardiac rhythm and heart failure devices, the company announced October 13.
The approval allows current patients with Medtronic's SureScan MR-conditional pacemakers, implantable cardioverter-defibrillators, and cardiac resynchronization therapy-defibrillators and leads to undergo 1.5 and 3T MRI scans anywhere in their body.
MRI is an important imaging technology used to diagnose conditions such as stroke, cancer, Alzheimer's disease, and muscle, bone and joint pain. However until now only 1% of all the cardiac patients with implanted devices could benefit from prescribed MRI scans because of the danger resulting from device incompatibility. FDA MRI compatibility approval now allows Medtronic to offer more options to ICD patients including those undergoing device replacement surgery.
As the population ages, the use of implantable devices such as pacemakers is growing. The estimate is that there are 5 million patients worldwide who currently are implanted with a pacemaker or implantable cardioverter-defibrillator. Simultaneously, the use of MRI as a diagnostic tool is also increasing, with an estimated millions of scans performed yearly.
Individuals over age 65 are twice as likely to need an MRI compared with younger patients, and between 50 and 75% of patients with electronic cardiac devices will likely need an MRI over their device's lifetime. Until now, this was a very high risk proposition. This FDA approval opens up a new life-saving option. Medtronic boasts that it is the first company in the U.S. to receive this FDA approval for MRI compatible cardiac implant devices, which should give it an edge over competitors in the cardio devices sector such as St. Jude Medical and Boston Scientific.
Marc Silver, MD, cardiologist at WakeMed Heart and vascular Physicians in Raleigh, NC, said, "The potential interaction between cardiac devices and MRIs has been a long-running concern for patients and physicians. Fortunately, advancements in MR-conditional cardiac device technology give patients more access to this important diagnostic tool."
While 1.5T scanners still comprise the majority of MRI installations, 3T scanners are expected to comprise more than half of new units--with some centers having only 3T scanners--since they offer faster scans and higher resolution images. Approval for MRI conditional scanning at both 1.5 and 3T allows patients to have improved access to MRI at a time and place most appropriate for their care. With 3T scanning, physicians and radiologists gain a clearer look into soft tissues, particularly critical when diagnosing serious conditions, often involving the brain and spine.
Joyce Laird is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
[Image courtesy of Medtronic]
About the Author(s)
You May Also Like