April 11, 2016
| vs. | ||
|
|
|
|
Describe your device and how it will benefit healthcare. | NERv is developing an implantable biochip to monitor a patient's health after surgery. The biochip is capable of detecting post-operative complications in real-time. The biochip is designed to target surgeries in the abdominal region, such as operations in the urinary and digestive systems. The biochip is very small, provides instantaneous feedback, and is made entirely out of biocompatible materials, making it completely harmless to the body. The biochip's biosensors continuously gather data about the body and analyze them. If an unexpected change happens, the sensor analyzes the change and sends feedback to the physician. The data is sent to a receiver wirelessly. The receiver is located in the trans-dermal patch placed on the wound after surgery. The receiver then transmits the data to the physician in order to determine if a complication is about to happen. Concurrently, the receiver alerts the patient if a complication is detected in order to seek medical attention immediately. |
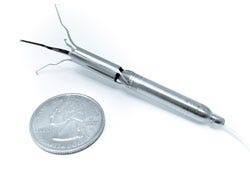
| Procyrion Inc. is working to give chronic heart failure patients a solution by developing the world's first catheter-deployed circulatory assist device intended for ambulatory use. The Aortix system, built around a powerful micromotor mounted on a set of expandable struts, is designed to rest the heart by reducing afterload and improve blood flow to vital organs. Unlike other circulatory assist devices, including ventricular assist devices (VADs), that involve invasive, high risk surgical procedures, Aortix is small enough--6 mm wide and less than 6.5 cm long--to allow for deployment without surgery and with minimal procedure risk. In a simple 20-minute procedure, a cardiologist could deliver Aortix through a catheter in the femoral artery to the descending thoracic aorta, a strategic location downstream of the heart that allows for combined benefit to the heart, kidneys, and other vital organs. Once the catheter sheath is in place, it is retracted to deploy the self-expanding nickel-titanium struts that anchor the pump to the aortic wall. |
How does your product differ from the competition? | Our technology is a disruptive one and thus there are no real competitors in our field. The nearest form of technology that provides value similar to ours is the medical imaging technology currently on the market, such as CT scans, MRIs, and radiography. These imaging techniques require the patient to show signs of sickness, before they can be used to identify the type and location of complications. However, they still fail 30% of the time (according to the Canadian Association of Radiologists). Our technology is predictive, real-time, and more efficient than the current alternatives on the market, promising to decrease the cost of inpatient healthcare. |
| This reversible anchoring is essential to what makes Aortix the first catheter-based pump suitable for ambulatory use in a walking, active patient. Additionally, the pump's location minimizes the common VAD surgical risks and potential for thrombotic stroke. In contrast to traditional VADs, where failure is often fatal, Aortix does not obstruct native blood flow and device failure is not life threatening. Aortix is designed to augment the natural function of the heart by accelerating a portion of the body's native blood flow within the micropump and directing it downstream in a series of "jets" that entrain and accelerate the native flow. In pre-clinical animal studies, Aortix increased the amount of blood the heart pumps by 10-15% while simultaneously lowering its energy needs by almost 40%. The end result is a nearly 60% increase in efficiency, and a heart that is working at a sustainable level while providing healthy blood flow and pressure to end organs. This groundbreaking interventional cardiology tool, conceived by cardiologist Dr. Reynolds M. Delgado, III, medical director of Mechanical Support Devices in Heart Failure at the Texas Heart Institute, has the potential to replace high-risk surgical devices and lengthy hospital stays with a lower-risk outpatient cardiology procedure. This type of paradigm shift could not only improve the quality of life for millions of chronic heart failure patients, but also benefit hospitals and payers by potentially cutting treatment costs and readmission rates. |
Do you have customers yet? | Our product will be tested in animals within the next six months. NERv has created a unique business model, where it will be targeting the veterinary market in order to prove clinical and commercial value for its product, before beginning clinical testing in humans. |
| Procyrion is a development-stage company, and the Aortix micro-pump is currently in preclinical testing to support a first-in-human trial scheduled for later this year (2016). Aortix is not approved for sale or use. |
How much money have you raised? | NERv has raised more than $100,000 in nondilutive capital. NERv has also raised approximately $125,000 in in-kind services from institutions in Ontario and throughout Canada. |
| $13.5 million |
Who are your investors? | -- |
| Fannin Innovation Studio, Scientific Health Development, undisclosed strategic and angel investors |
What is the next milestone for your device? | The next milestone will be to begin testing the biochip in animals to prove clinical and commercial value. |
| First-in-Human clinical trial scheduled for later in 2016. |
Continue to "10 Digital Health and Medtech Startups that Shone at SXSW"
You May Also Like