April 11, 2016
| vs. | ||
|
|
|
|
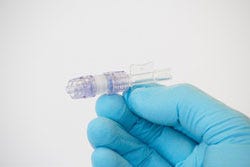
Describe your device and how it will benefit healthcare. | Clinical studies show that 7% of all I.V. lines are dislodged. In the United States alone, there are over 200 million I.V. lines placed annually. I.V. Dislodgment is estimated to cost the U.S. healthcare system nearly $1 billion a year. We are developing a new device to prevent catheter dislodgment called the SafeBreak IV. The current standard for preventing IV dislodgment is the use of tape or more expensive securement devices. While adhesives and securement are important, nothing can prevent dislodgment when a large force is applied to an IV line. The SafeBreak IV fits in-between the patient's catheter and the IV tubing, and creates a sealed liquid connection. Its design allows moderate pull force but separates before the adhesive fails, keeping harmful trauma from being delivered to the patient's IV site. When the device separates, its patented technology prevents blood loss and triggers the IV pump's high pressure alarm to alert the nurse. Replacing a SafeBreak IV is painless and takes seconds, so the patient can quickly resume their medication therapy without having to experience the pain of a dislodged IV and the reinsertion of a new one. The nursing staff can spend their time working on value-added activities and the hospital saves money on supplies, labor, and patient satisfaction. |
| VuVa Magnetic Vaginal Dilators are a new, nonsurgical approach to helping millions of women suffering from multiple pelvic pain conditions and sexual discomfort. With more than 6 million women suffering from vulvodynia in the United States alone, women are looking for safe and effective treatments. In a double-blind, placebo clinical trial, VuVa was shown to reduce 80% of subjects' vulvar pain with magnetic dilators. VuVa Magnetic Dilator therapy can also be incorporated into treatment plans for other conditions, such as vaginismus, vulvar vestibulitis, dyspareunia, menopause, vaginal stenosis, vaginal atrophy, and vaginal dryness. Many women suffering from these pelvic pain conditions have been placed on heavy pain medications, anti-depressants, or have been advised to have invasive surgeries with low success rates. VuVa Dilators offer a low-cost, safe, noninvasive treatment option to renew the sexual desire and comfort these women deserve. VuVa Dilators are made in the United States. |
How does your product differ from the competition? | Adhesives, tapes, and expensive securement devices have long been viewed as the last lines of defense in avoiding IV complications. While basic securement is essential, all securement devices will fail eventually, resulting in catheter dislodgment. Unlike basic securement devices, the SafeBreak IV creates a necessary separation in the IV line, allowing us to protect the IV catheter during extreme tension. The SafeBreak IV helps understaffed nurses avoid stressful and time consuming IV dislodgment, and keeps patients progressing through the continuum of care.
|
| VuVa Dilators have strategically placed Neodymium magnets inside each dilator, unlike all other dilators on the market, which are simply made up of silicone or plastic. The patent-pending neodymium component consists of more than 60 neodymium magnets in a set of five graduated dilators. The negative magnetic field allows an increase in blood flow to the muscles and ligaments in the vagina, relaxing the painful areas and calming the nerves. Patients have reported that by using the VuVa Dilator twenty minutes before sex, dilators can decrease penetration and intercourse pain significantly. In addition to VuVa reducing pelvic pain, subjects suffering from vaginal dryness due to menopause have reported an additional benefit of dilators helping to create natural lubrication. It is thought that future clinical trials will prove by the increase of blood flow to the area, estrogen is created, eliminating the need for expensive and controversial estrogen treatments. |
Do you have customers yet? | The device is not yet cleared for sale by FDA, but we will start our first clinical trial in the fall of 2016. |
| Since VuVa Dilators became available on the market in late 2015, we have only used social media, Google ads, and pelvic pain forums to direct traffic to our website. After selling more than 1000 dilators and receiving positive feedback, it validated our decision to perform clinical trials. Clinical trials have provided positive results by reducing nerve pain in subjects--so much so that each subject purchased a set of VuVa dilators at the conclusion of the study. Our story and product have appeared on SELF Magazine online, Natural Awakening Magazine, Yahoo Española online, and other online publications, which is driving traffic to our site. At the current time we are shipping worldwide, and are in discussions with medical device distributors in the United States and South America. |
How much money have you raised? | Approximately $900K |
| $250,000 |
Who are your investors? | Zero To 510 Medical Device Accelerator, venture capital firms, and angel investors |
| At the present time, the total investment has been provided to the two coowners and the inventors of the product, Tara Langdale Schmidt and Robert Smithson. |
What is the next milestone for your device? | FDA 510(k) clearance is the next milestone for our device. We will be submitting our 510(k) in the fall of 2016. |
| We are seeking to have clinical trials performed in larger cities to show efficacy of using VuVa Dilators in the treatment of other pelvic pain conditions. At the completion of these clinical trials, we are confident they will help us gain FDA approval for the magnetic component in our dilators. Vaginal dilators are an already approved Class II medical device. We are searching for reputable distributors in Europe and other countries, as pelvic pain is a worldwide silent epidemic. We are evaluating other investment opportunities to raise capital to facilitate further clinical trials and marketing. |
You May Also Like