Procyrionvs.PhotoniCare
April 4, 2016
| vs. | ||
|
|
|
|
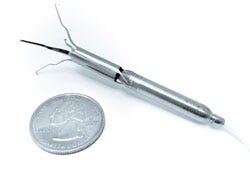
Describe your device and how it will benefit healthcare. | Procyrion Inc. is working to give chronic heart failure patients a solution by developing the world's first catheter-deployed circulatory assist device intended for ambulatory use. The Aortix system, built around a powerful micromotor mounted on a set of expandable struts, is designed to rest the heart by reducing afterload and improve blood flow to vital organs. Unlike other circulatory assist devices, including ventricular assist devices (VADs), that involve invasive, high risk surgical procedures, Aortix is small enough--6 mm wide and less than 6.5 cm long--to allow for deployment without surgery and with minimal procedure risk. In a simple 20-minute procedure, a cardiologist could deliver Aortix through a catheter in the femoral artery to the descending thoracic aorta, a strategic location downstream of the heart that allows for combined benefit to the heart, kidneys, and other vital organs. Once the catheter sheath is in place, it is retracted to deploy the self-expanding nickel-titanium struts that anchor the pump to the aortic wall. |
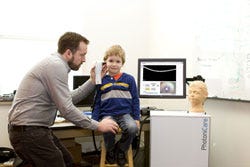
| Nearly every person on the planet has experienced an ear infection. They are painful and the bane of parents across the globe. They are the leading cause of hearing loss and surgery in children, and responsible for estimated annual costs of more than $10 billion and 30 million office visits in the United States. alone. Currently, the average physician misdiagnoses these infections up to 50% of the time, but that information is still used to determine antibiotic treatment and surgical intervention. We have developed the ClearView, a handheld imaging device that will help physicians quickly, easily, and accurately diagnose ear infections. We want to get children the appropriate treatment as soon as possible, avoiding months of repeat office visits, ineffective antibiotics, and poor quality of life. |
How does your product differ from the competition? | This reversible anchoring is essential to what makes Aortix the first catheter-based pump suitable for ambulatory use in a walking, active patient. Additionally, the pump's location minimizes the common VAD surgical risks and potential for thrombotic stroke. In contrast to traditional VADs, where failure is often fatal, Aortix does not obstruct native blood flow and device failure is not life threatening. Aortix is designed to augment the natural function of the heart by accelerating a portion of the body's native blood flow within the micropump and directing it downstream in a series of "jets" that entrain and accelerate the native flow. In pre-clinical animal studies, Aortix increased the amount of blood the heart pumps by 10-15% while simultaneously lowering its energy needs by almost 40%. The end result is a nearly 60% increase in efficiency, and a heart that is working at a sustainable level while providing healthy blood flow and pressure to end organs. This groundbreaking interventional cardiology tool, conceived by cardiologist Dr. Reynolds M. Delgado, III, medical director of Mechanical Support Devices in Heart Failure at the Texas Heart Institute, has the potential to replace high-risk surgical devices and lengthy hospital stays with a lower-risk outpatient cardiology procedure. This type of paradigm shift could not only improve the quality of life for millions of chronic heart failure patients, but also benefit hospitals and payers by potentially cutting treatment costs and readmission rates. |
| Ear infections are currently diagnosed by looking at the surface of the eardrum with a magnifying glass (i.e., the otoscope). However, the infection is in the middle ear, which cannot be visualized with current technology. PhotoniCare's ClearView solves this limitation by simply looking through the eardrum and into the middle ear. The ClearView works like ultrasound imaging, except we use near-infrared light instead of sound to produce cell-level resolution, 3-D images of what's going on behind the eardrum and the infection hiding there. For the first time, we can visualize and characterize key features in the middle ear, like effusions (fluid) and biofilms (linked to tube surgery). Physicians can use this information to get children the care they need immediately. There are other established technologies that aim to improve diagnosis of ear infections, such as tympanometry and acoustic reflectometry, but these techniques are difficult to use and/or have poor diagnostic abilities. ClearView is the only technology that can see into the middle ear. |
Do you have customers yet? | Procyrion is a development-stage company, and the Aortix micro-pump is currently in preclinical testing to support a first-in-human trial scheduled for later this year (2016). Aortix is not approved for sale or use. |
| Over the past couple of years, we've piloted our technology on more than 200 patients. Our product is currently in use in an ongoing pilot clinical study in Children's National Health System, a top pediatric hospital in Washington, DC. We are currently looking to expand our clinical trial sites to other parts of the country, or perhaps international clinical sites. |
How much money have you raised? | $13.5 million |
| This technology was prototyped and validated in the academic lab of PhotoniCare's founders, where more than $5 million was leveraged to advance it to the clinical stage. In PhotoniCare, we have raised nearly $2.5 million from a mix of private capital and federal grants. |
Who are your investors? | Fannin Innovation Studio, Scientific Health Development, undisclosed strategic and angel investors |
| Our investor team includes a mix of institutional and angel money. In addition to capital, they also bring extensive expertise in medical device development, regulatory strategy, reimbursement strategy, and several successful exits. |
What is the next milestone for your device? | First-in-Human clinical trial scheduled for later in 2016. |
| Our next big milestones are prototype finalization and design freeze, FDA 510(k) approval, and a Series A raise planned in 2016 to launch our first product in 2017. |
Continue to "10 Digital Health and Medtech Startups that Shone at SXSW"
You May Also Like