Respirixvs.PhotoniCare
March 28, 2016
| vs. | ||
|
|
|
|
Describe your device and how it will benefit healthcare. | Cardiospire is a portable, handheld device for noninvasive monitoring of congestive heart failure (HF) patients. The device extracts hemodynamic parameters from naturally expired airflow. Our patented innovations allow us to obtain reproducible measurements independent of interpatient variability with little to no subject training required. Cardiospire indicates when it has successfully taken measurements and electronically transmits the processed data to the patient's physician's office. The data may then be used to tailor heart failure drug dosing to correct fluid balance and optimize cardiac function in order to prevent hospitalizations. The potential impact is significant, as HF is the leading cause of hospitalization in patients over 65 and 25% of patients are readmitted within 30 days. |
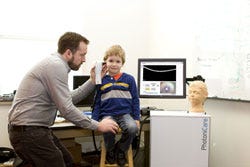
| Nearly every person on the planet has experienced an ear infection. They are painful and the bane of parents across the globe. They are the leading cause of hearing loss and surgery in children, and responsible for estimated annual costs of more than $10 billion and 30 million office visits in the United States. alone. Currently, the average physician misdiagnoses these infections up to 50% of the time, but that information is still used to determine antibiotic treatment and surgical intervention. We have developed the ClearView, a handheld imaging device that will help physicians quickly, easily, and accurately diagnose ear infections. We want to get children the appropriate treatment as soon as possible, avoiding months of repeat office visits, ineffective antibiotics, and poor quality of life. |
How does your product differ from the competition? | The standard method of monitoring daily weights has been shown to be inadequate for keeping HF patients out of the hospital. Recently, CardioMEMS from St. Jude Medical has entered the market, but it requires surgery to implant the $17,000 device. Also, its price has been scrutinized by payers and independent third parties such as the Institute for Clinical and Economic Review. Similar to CardioMEMS, Cardiospire has been designed for at-home monitoring of cardiac function to detect early symptoms of acute decompensation. Our patented technology allows extraction of the same biometric signals plus additional parameters from the hands and respiration. While previous studies have shown that remote monitoring of cardiac function with physician feedback can reduce hospitalizations and improve quality of life, ours is the first device that can perform this function economically and non-invasively. This will substantially improve the cost-effectiveness of remote heart failure monitoring and eliminate many of the hurdles facing CardioMEMS, such as resistance from patients to have an implant and from payers to reimburse for the device. |
| Ear infections are currently diagnosed by looking at the surface of the eardrum with a magnifying glass (i.e., the otoscope). However, the infection is in the middle ear, which cannot be visualized with current technology. PhotoniCare's ClearView solves this limitation by simply looking through the eardrum and into the middle ear. The ClearView works like ultrasound imaging, except we use near-infrared light instead of sound to produce cell-level resolution, 3-D images of what's going on behind the eardrum and the infection hiding there. For the first time, we can visualize and characterize key features in the middle ear, like effusions (fluid) and biofilms (linked to tube surgery). Physicians can use this information to get children the care they need immediately. There are other established technologies that aim to improve diagnosis of ear infections, such as tympanometry and acoustic reflectometry, but these techniques are difficult to use and/or have poor diagnostic abilities. ClearView is the only technology that can see into the middle ear. |
Do you have customers yet? | Respirix has completed a pilot study of its Cardiospire device and is currently entering a pivotal clinical trial in the United States. |
| Over the past couple of years, we've piloted our technology on more than 200 patients. Our product is currently in use in an ongoing pilot clinical study in Children's National Health System, a top pediatric hospital in Washington, DC. We are currently looking to expand our clinical trial sites to other parts of the country, or perhaps international clinical sites. |
How much money have you raised? | Approximately $1 million Series A |
| This technology was prototyped and validated in the academic lab of PhotoniCare's founders, where more than $5 million was leveraged to advance it to the clinical stage. In PhotoniCare, we have raised nearly $2.5 million from a mix of private capital and federal grants. |
Who are your investors? | High net-worth individuals and institutional investors |
| Our investor team includes a mix of institutional and angel money. In addition to capital, they also bring extensive expertise in medical device development, regulatory strategy, reimbursement strategy, and several successful exits. |
What is the next milestone for your device? | Clinical trials in HF patients |
| Our next big milestones are prototype finalization and design freeze, FDA 510(k) approval, and a Series A raise planned in 2016 to launch our first product in 2017. |
Continue to "10 Digital Health and Medtech Startups that Shone at SXSW"
You May Also Like