Procyrionvs.BioAnalytics
March 28, 2016
| vs. | ||
|
|
|
|
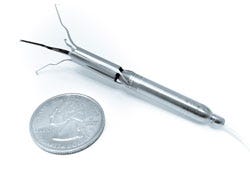
Describe your device and how it will benefit healthcare. | Procyrion Inc. is working to give chronic heart failure patients a solution by developing the world’s first catheter-deployed circulatory assist device intended for ambulatory use. The Aortix system, built around a powerful micromotor mounted on a set of expandable struts, is designed to rest the heart by reducing afterload and improve blood flow to vital organs. Unlike other circulatory assist devices, including ventricular assist devices (VADs), that involve invasive, high risk surgical procedures, Aortix is small enough—6 mm wide and less than 6.5 cm long—to allow for deployment without surgery and with minimal procedure risk. In a simple 20-minute procedure, a cardiologist could deliver Aortix through a catheter in the femoral artery to the descending thoracic aorta, a strategic location downstream of the heart that allows for combined benefit to the heart, kidneys, and other vital organs. Once the catheter sheath is in place, it is retracted to deploy the self-expanding nickel-titanium struts that anchor the pump to the aortic wall. |
| The Medical Acute Immune Diagnostic (MedAID) system is a broadly applicable technology which reduces immunoassay complexity and cross reactivity to provide tests with faster turnaround times, fewer steps, decreased technician time requirement, easier to automate point-of-care devices, less variability, higher sensitivity, and increased capacity for multiplexing. This is achieved through the use of a proprietary immune complex, which allows for multiple steps to occur in tandem. To our knowledge, this system is the world’s first universally applicable, label based, single-step, sandwich immunoassay. |
How does your product differ from the competition? | This reversible anchoring is essential to what makes Aortix the first catheter-based pump suitable for ambulatory use in a walking, active patient. Additionally, the pump’s location minimizes the common VAD surgical risks and potential for thrombotic stroke. In contrast to traditional VADs, where failure is often fatal, Aortix does not obstruct native blood flow and device failure is not life threatening. Aortix is designed to augment the natural function of the heart by accelerating a portion of the body’s native blood flow within the micropump and directing it downstream in a series of “jets” that entrain and accelerate the native flow. In pre-clinical animal studies, Aortix increased the amount of blood the heart pumps by 10-15% while simultaneously lowering its energy needs by almost 40%. The end result is a nearly 60% increase in efficiency, and a heart that is working at a sustainable level while providing healthy blood flow and pressure to end organs. This groundbreaking interventional cardiology tool, conceived by cardiologist Dr. Reynolds M. Delgado, III, medical director of Mechanical Support Devices in Heart Failure at the Texas Heart Institute, has the potential to replace high-risk surgical devices and lengthy hospital stays with a lower-risk outpatient cardiology procedure. This type of paradigm shift could not only improve the quality of life for millions of chronic heart failure patients, but also benefit hospitals and payers by potentially cutting treatment costs and readmission rates. |
| The MedAID system was developed using an alternative design of the molecular components already used in sandwich immunoassays. BioAnalytics technology is applicable to nearly all existing platforms within the immunoassay industry, allowing existing tests to be modified to achieve the same benefits as BioAnalytics developed tests. This manifests in tests that are able to provide half the number of steps, two times faster turnaround times, 100 times higher sensitivity, 37 times improved specificity, and 390 times reduced cross-reactivity in multiplexing. |
Do you have customers yet? | Procyrion is a development-stage company, and the Aortix micro-pump is currently in preclinical testing to support a first-in-human trial scheduled for later this year (2016). Aortix is not approved for sale or use. |
| BioAnalytics is currently prerevenue. To date BioAnalytics initiated an alpha prototype sale to a research group for early evaluation and validation. Through the National Science Foundation I-CORPs program, BioAnalytics has discussed the MedAID technology with more than 100 interested industry and academic professionals including researchers, purchasing agents, and clinicians from academic/federal research and hospital laboratories as well as drug-discovery and pharmaceutical companies. BioAnalytics will begin initial sales in the later stages of 2017. |
How much money have you raised? | $13.5 million |
| Approximately $250,000 through both dilutive and nondilutive means |
Who are your investors? | Fannin Innovation Studio, Scientific Health Development, undisclosed strategic and angel investors |
| Primarily founders and friends/family. BioAnalytics was awarded a $150,000 Phase I NSF SBIR in January 2015 and recently applied for a Phase II application (pending as of March 2016). |
What is the next milestone for your device? | First-in-Human clinical trial scheduled for later in 2016. |
| BioAnalytics next major milestone will be the introduction of it’s first product portfolio of 10 cytokine ELISA kits. To achieve this, the team will optimize protocols for manufacturing with currently selected OEMs as well as validate the manufacturing processes to be employed for large scale sales. Additionally, BioAnalytics will be optimizing the sensitivity component of it’s immunoassay technology in order to lower the limit of detection, or sensitivity benefits, to fg/ml instead of the currently accepted pg/ml limits. The last milestone set for the year will be to strengthen the patent portfolio through the use of a Continuation-in-Part application as well as three divisional applications. |
You May Also Like