Describe your device and how it will benefit healthcare.
March 28, 2016
| vs. | ||
|
|
|
|
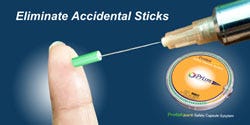
Describe your device and how it will benefit healthcare. | The ProteXsure Safety Capsule System aims to eliminate and/or greatly reduce needle sticks. The system is a patented, disposable, rotating cartridge filled with 100 needle covers that automatically turns after every single use via an internal spring. |
| Vestagen's VESTEX enhanced fabric is designed to improve the safety of healthcare workers and their patients. According to the Bureau of Labor Statistics, healthcare professionals experience the highest rates of injury and illness of any occupation in the United States. Frequent exposure to body fluid splatters and spills result in bacterial colonization of apparel, a well-documented source of pathogen transmission. Pathogen transmission is linked to worker illness and high rates of healthcare-associated infections (HAIs) in patients. The cost of HAIs is high-thousands of lost lives, millions of additional hospital days, and billions of dollars annually. Vestagen currently manufactures healthcare worker uniforms and outerwear (scrubs, lab coats, etc.) with VESTEX technology. VESTEX garments are engineered to have robust liquid repellency and antimicrobial properties, along with breathability, comfort, and durability. |
How does your product differ from the competition? | The device is a patented sharps safety device that will redefine needle safety protocol. It is simple (nonintegral) and is the most cost-effective sharps safety device on the market. No competitive product comes close to doing what this product does. |
| VESTEX is the first and only apparel product to earn the exclusive endorsement of the American Hospital Association. VESTEX is proven in a clinical setting to reduce the acquisition and retention of contaminants on clothing. In a peer-reviewed published study, researchers reported a statistically significant MRSA reduction of more than 99.99% on the VESTEX scrubs compared with standard uniforms. |
Do you have customers yet? | Yes, we just received our CE Mark and are planning a summer rollout with our global distributor to more than 400,000 dentists and hundreds of thousands of healthcare professionals in Europe. |
| VESTEX is currently being purchased by healthcare systems and providers. In any given month, Vestagen receives hundreds of orders from approximately 10-15 different customers. Vestagen is in late-stage negotiations with large health systems in the Mountain West, Southeast and Northeast portions of the United States. |
How much money have you raised? | Self-funded |
| More than $10 million |
Who are your investors? | NA |
| Vestagen is backed by an elite group of venture and private investors, including Advent Life Sciences, Sofinnova Healthquest Partners L.P., V-Ten Capital - VVEST LLC, Merefin Capital, and ClearWell Group. |
What is the next milestone for your device? | FDA 510(k) is next. We have our CE Mark. |
| VESTEX fabric is currently registered with FDA as a Class I medical device, and the company plans to file for FDA clearance as a Class II medical device in 2016. |
You May Also Like