Surgical Perspectivevs.Briteseed
March 23, 2015
| vs. | ||
|
|
|
|
Describe your device and how it will benefit healthcare. | Surgical Perspective is dedicated to development of a full range of single-use retractors for abdominal surgery. We aim to improve the outcome and costs of surgery by decreasing the number of incisions without compromising the quality and security of organ exposure. |
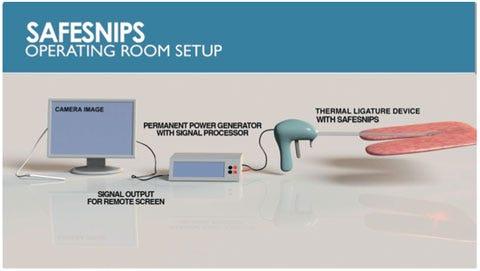
| The SafeSnips patented, intraoperative platform technology can be integrated into existing surgical cutting tools to facilitate the real-time detection of blood vessels before surgical cuts are made. U.S. hospitals lose billions of dollars in unreimbursed costs due to inadvertent cuts into vasculature each year. Patients who suffer from these cuts face a mortality rate of up to 32%, and those who survive face an increased hospitalization time of nine days and $210,000 in added costs of care. |
How does your product differ from the competition? | We offer an extra hand inside the abdomen of the patient without any extra trocar, limiting instruments conflicts. |
| To date, no company has seamlessly integrated blood vessel detection technology into existing cutting tools. Instead, to locate a vessel during surgery, a surgeon must use a separate laparoscopic Doppler probe or perform intraoperative imaging, such as CT or MRI. All of these alternatives require added steps, which lengthen surgery times and thereby increase the risk of complication. Further, Doppler technology requires surgeons to alter their behavior while imaging alternatives are costly, require contrast agents, and can result in exposure to radiation. |
Do you have customers yet? | Started sales in Europe and the United States |
| We are at the prerevenue stage. For future clinical testing, we have identified and will engage the chair of the department of surgery and Loyal and Edith Davis professor of surgery Dr. Nathaniel Soper of Northwestern University to participate as a trial investigator. We will focus on out-licensing this single-use technology across a platform of applications. Initial target customers will be manufacturers of thermal ligature devices (a $1.2-billion global market) and end users will be surgeons who perform minimally invasive general and gynecological surgeries. We have already had partnership and licensing discussions with four major medical device companies, including Covidien, Intuitive Surgical, and Novadaq. |
How much money have you raised? | $1.5 million |
| $46,250 in cofounder contributions; significant business plan awards, including $73,500 in cash; $1.15 million raised in seed round using a convertible note |
Who are your investors? | -- |
| Lead investors include the Evansville, Indiana, Angel Invstors and Grand Order of Successful Entrepreneurs (supported by Dr. Jack Gill, cofounder of Vanguard Ventures); other investors include physicians who would serve as potential end users and domain experienced investors who have spent more than 40 years in the medical device space. |
What is the next milestone for your device? | Expand sales throughout the United States and gain market access in Asia |
| Refine the current prototype for a pilot study planned at Northwestern University in support of FDA 510(k) application. This includes miniaturization of the SafeSnips technology to fit within a 5-mm trocar, which is expected later this year. |
You May Also Like