The lawsuit, filed in Los Angeles, seeks millions of dollars in damages from Johnson & Johnson, claiming that a company it acquired knowingly marketed an unproven, off-label use for its sinus balloon catheters.
Brian Buntz and Chris Newmarker
|
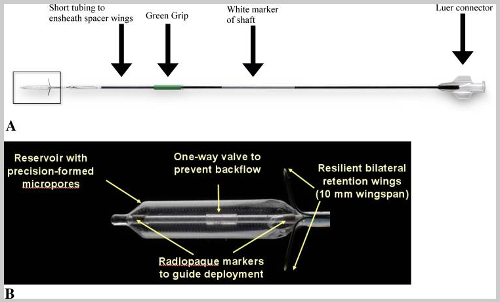
This image was pulled from a 2011 clinical study describing the The MicroFlow spacer as "A Drug-Eluting Stent for the Ethmoid Sinus." The image shows the device's reservoir and shaft in "A" and the reservoir's features in "B." |
Acclarent, a startup acquired by Johnson & Johnson in 2010, knowingly engaged in off-label promotion of its Relieva Stratus MicroFlow spacer for an unproven drug delivery use, according to a former employee's whistleblower complaint filed in Los Angeles Superior Court.
The new lawsuit, State of California ex rel. Melayna Lokosky vs. Acclarent, Inc.; Ethicon, Inc.; and Johnson & Johnson, maintains that J&J, which acquired Acclarent for $785 million in 2010, is culpable for millions of dollars in damages, as insurance companies were led to pay for Acclarent devices that were misbranded, medically unnecessary, and marketed to physicians for unproven off-label uses.
Two former top Acclarent executives--William Facteau of Atherton, CA, who was CEO, and Patrick Fabian of Lake Elmo, MN, who was vice president of sales--already face a variety of federal conspiracy and wire fraud charges in Massachusetts. The federal grand jury indictment accuses them of marketing Acclarent's Relieva Stratus Microflow Spacer for off-label use--for delivering steroids rather than simply opening sinuses. The indictment states that the sinus spacer had in fact been designed for the purpose of delivering Kenalog-40 steroid through small holes in the balloon, yet deceived FDA about the intended use of the device.
After being taken into federal custody, Facteau and Fabian have been released after posting $5-million bond and $500,000 bond, respectively.
A patent filed for the device in 2004, US7361168 B2, describes the technology as a "transnasally insertable implantable substance delivery device for delivering a diagnostic or therapeutic substance to a location within a paranasal sinus or paranasal sinus ostium of a human or animal subject."
According to the aforementioned federal case, Acclarent had planned from the beginning to develop the Stratus device "to provide sustained release of the steroid Kenalog-40 in the nasal passage" adding that it "did not elute saline for any significant period of time." The federal indictment goes on to say that, because the pores of the device were designed to elute Kenalog-40 rather than saline, "most of the saline would run right out of the device" when the device was used according to the indication for which it won FDA clearance
The California lawsuit takes accusations further than the federal case, stating that Acclarent "has known for years that the MicroFlow Spacer loaded with Kenalog-40--a corticosteroid approved only for injection in joints or muscle tissue, and specifically not approved for uses in sinuses--provided no additional benefits to patients." Kenalog-40 is a suspension drug and thus must be injected into tissue to be viable.
The lawsuit further alleges that the company sought to avoid revealing that the technology was ineffective publicly, and changed the protocol of its sole clinical trial midstream. "Had Acclarent been truthful with the FDA, the FDA would never have allowed the MicroFlow Spacer to be brought to market for Acclarent's intended (but unapproved) drug-delivery use," the suit continues.
A 510(k) application for the device was initially received by FDA on August 23, 2006. After a decision made by FDA regarding the application on September 15, 2006, Acclarent filed a new application under a new number. FDA cleared the device for sale on October 2, 2008 "as a postoperative spacer to maintain an opening to the ethmoid sinus within the first 14 days following surgery."
A spokesman for Acclarent could not be immediately reached for comment regarding the litigation. Facteau and Fabian, the former Acclarent executives named in the federal case, are not specifically named in this lawsuit. Leo Cunningham, a lawyer at Wilson Sonsini Goodrich & Rosati representing Facteau in the federal case, declined to comment regarding this matter. Lawyers for Fabian could not be immediately reached.
|
Melayna Lokosky is the whistleblower in a recent lawsuit against J&J's Acclarent division. |
The lawsuit in Los Angeles also repeats the accusations leveled in the federal indictment in Massachusetts, that Acclarent lied to FDA when submitting a 510(k) application to market the device, claiming the device was intended for use to deliver saline to a patient's nasal cavities for no more than 14 days, when its intended use was to deliver a steroid to the sinus cavities for 28 days or longer. Achieving approval from the FDA for the drug-delivery application would have required using the considerably lengthier and costlier PMA pathway to market.
The federal indictment mentions cases in which the physicians experienced troubles with the initial Stratus device launched in 2008, including it migrating or slipping out or its retention wings detaching and remaining in patients. Acclarent launched a physically modified version of the Stratus device in 2009, though it too only had 510(k) clearance for opening the frontal sinus post-operatively and moistening with saline.
Acclarent, and its incubator Exploramed II, were named in a 2006 patent infringement suit brought by Quest Medical Inc. Quest Medical maintained it had originally had the idea for a balloon sinuplasty device. That case was eventually settled out of court.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)

