January 8, 2016
A growing number of patients are filing lawsuits against CR Bard, alleging that the company's IVC filters are dangerous.
Brian Buntz
|
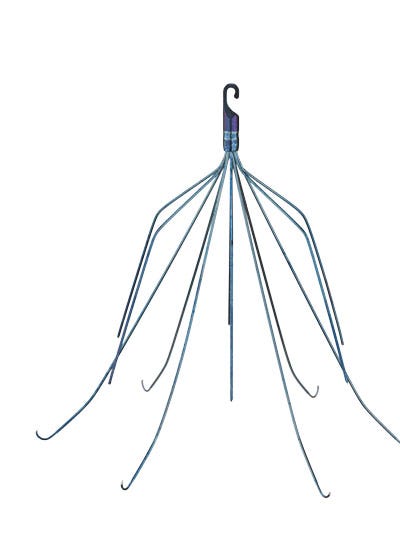
Both CR Bard's Recovery and G2 filters (the latter is pictured here) were placed inside the placed inside the inferior vena cava to catch blood clots. |
There has been a considerable amount of negative news related to CR Bard's vena cava filters on the heels of a year-long NBC investigation. Most recently, there has been an uptick in litigation related to the devices, which can potentially migrate within patients, possibly puncturing the vena cava.
Judge David G. Campbell of the U.S. District Court, District of Arizona has approved multidistrict litigation related to the Bard Recovery, Bard G2, and other Bard IVC filters that have been filed from August to recently.
While there are 72 cases pending in the Bard litigation, the volume of lawsuits against the company could ultimately be several thousand.
In Mississippi, plaintiff Alec Caldwell has filed a product liability and personal injury lawsuit related to another Bard product--the Eclipse inferior vena cava filter. He claims the device became severely tilted and that doctors are unable to remove the device.
NBC had alleged that the company was aware that its G2 and Recovery filters were unsafe, but continued to sell them. The network obtained a report from an independent doctor concluding that its Recovery filter had "an 11.5 times higher reporting rate for filter embolization deaths compared with all other vena cava filters."
NBC states that the Recovery filter had been linked with more than 300 injuries and 27 deaths.
Last year, a former regulatory affairs professional at Bard, Kay Fuller, announced that her signature had been forged on the regulatory documents related to the Recovery device.
While the G2 was intended to address problems with the Recovery device, at least a dozen deaths have been linked to the device.
In 2013, the company settled separate litigation related to its vaginal mesh products for an undisclosed amount and, last year, the company settled an additional batch of related lawsuits for $200 million.
Bard also recently announced plans to close a plant in Minnesota, shedding 185 jobs.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
About the Author(s)
You May Also Like