July 12, 2017
The liquid biopsy, already a leap in diagnostic technology, is advancing further--this time from artificial intelligence (AI).
|
Dr. Jurgi Camblong, CEO and cofounder of SOPHiA GENETICS |
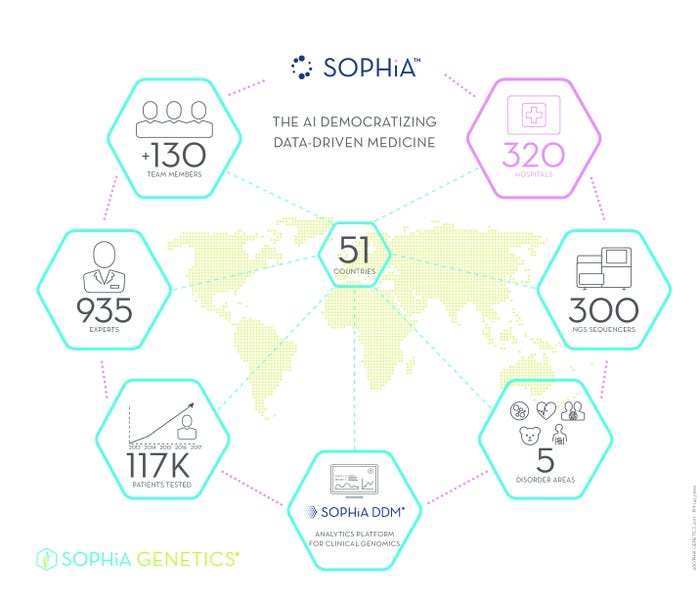
At the 2017 Annual Meeting of the American Society of Clinical Oncology, SOPHiA GENETICS unveiled a new solution powered by SOPHiA, the company's AI, to "help clinicians diagnose, treat, and monitor cancer earlier and more effectively by looking at circulating tumor DNA (ctDNA) contained in patients' liquid samples such as blood, urine, and cerebral spinal fluid," explained Dr. Jurgi Camblong, CEO and cofounder of SOPHiA GENETICS. The SOPHiA DDM analytical platform powered by SOPHiA AI is currently being used by more than 315 institutions in 51 countries, forming what Camblong calls "the world's largest clinical genomics community."
The liquid biopsy is "a new paradigm," Camblong told Qmed. "Compared to tissue biopsies, liquid biopsies allow clinicians to perform analysis of solid tumors and hematological malignancies at various time points to detect tumor progression and monitor treatments' effectiveness. Also, a liquid biopsy tends to be more accurate than a tissue biopsy as it offers a more comprehensive picture of the spread or remission of a cancer. To sum-up, liquid biopsies allow clinicians to have several different analyses run over time in one blood sample and adapt treatments accordingly. For patients, this new approach represents a faster and less invasive alternative."
There have been some challenges in liquid biopsy adoption, however, he said. "The lack of a standardized, accurate, and unbiased analytical solution able to take into account low ctDNA levels has for a long time been the main significant barrier to the widespread adoption of liquid biopsies in hospitals," Camblong said.
To address these barriers, SOPHiA provides a "standardized DNA analysis approach to liquid biopsy testing, built upon the network of over 315 hospitals from 51 countries already using SOPHiA for genomic data analysis," he explained. "Even with low ctDNA levels, SOPHiA provides indispensable insights into tumors' profiles, straight from liquid samples. Rather than waiting for months to detect changes on an imaging scan, SOPHiA allows clinicians to monitor a tumor's progression with remarkable precision from a simple blood test."
The company has also built OncoPortal, an interface that can analyze all the genetic variants detected by SOPHiA in ctDNA from solid tumors and hematological malignancies. "OncoPortal flags associations between human gene variants, disease causality, progression, drug efficacy, and toxicity to help the clinicians better leverage the data analyzed by SOPHiA in order to provide personalized care to patients," he said. "SOPHiA's application for liquid biopsies is also available for clinical trials, making it possible to identify the patients most likely to benefit from new treatments."
SOPHiA has already delivered on several milestones highlighted in President Obama's Precision Medicine Initiative, which aimed to connect hospitals, pool molecular data, and share knowledge, Camblong added. "The next step is now to expand our knowledge base and head towards a future of real-time epidemiology--an era when we can monitor treatments almost real-time within patients' cohorts and where we will be able to say that one particular patient's cancer is identical to that of 10,000 other patients, who had received treatment plan A and survived," he explained. "To do so, and particularly in oncology, we need to get access to data about cancer types, cancer stage, patients' treatments, and treatments' outcomes, which will allow us to cluster patients and further leverage previous diagnosis to inform the next ones, ensuring patients get even more personalized treatments. SOPHiA GENETICS already put the infrastructure in place and democratized its innovative approach. The next step is to have access to more and different types of data and metadata to expand our knowledge base and fully leverage our AI SOPHiA for Data-Driven Medicine."
|
Map courtesy SOPHiA GENETICS |
In April, the company celebrated the hundred thousandth patient genomic profile analyzed by SOPHiA, Camblong reported, and the company is working to grow that number significantly. "As pharma shifts to a 'value-based' pricing model, where reimbursement is tied to tangible patients' benefits, AI will play a key role to ensure treatments are targeted and personalized for each patient, building on the thousands of previous patient cases we will have analyzed," he said. "By 2020, our goal for SOPHiA is to have participated in diagnosing 1 million patients. By then, we should have succeeded in ensuring that patients get access to the best treatments, not based on clinical trials but rather building on evidence-based medicine. Moving forward, we see SOPHiA playing an even more important role in targeting treatments, based on the value and benefits they have previously proven to bring to patients."
Oncology should be the first disease area to benefit from this technological advance, he added. "I think AI will play a key role to ensure treatments are targeted and personalized for each patient and oncology should be the first disease area to benefit from this technological advance," Camblong said. "SOPHiA has been exposed to a critical mass of data and today participates in sharing knowledge and building a collective intelligence amongst hospitals to save patients' lives."
For more details, please view SOPHiA GENETICS's corporate video. The company is on Twitter at @JurgiCamblong and @SOPHiAGENETICS.
Don't miss the upcoming MD&M Minneapolis Conference and Expo, November 8-9, 2017.
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to MD&DI and Qmed. Reach her at [email protected] and on Twitter at @daphneallen
About the Author(s)
You May Also Like