July 11, 2016
Insightec's ExAblate Neuro uses focused ultrasound to destroy brain tissue in tiny areas thought to be causing tremors.
Qmed Staff
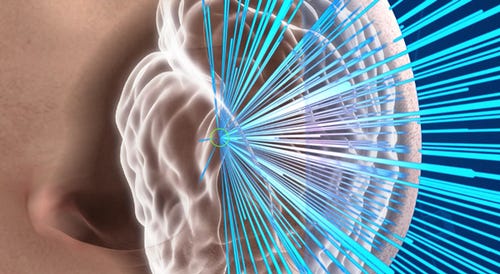
U.S. FDA said Monday that it has approved what it says is the first focused ultrasound device to treat essential tremor.
ExAblate Neuro, made by Insightec (Dallas), is meant for patients who have not responded to medication. It uses magnetic resonance (MR) images taken during the procedure to deliver focused ultrasound.
"Patients with essential tremor who have not seen improvement with medication now have a new treatment option that could help them to avoid more invasive surgical treatments," said Carlos Peña, PhD, director of the division of neurological and physical medicine devices in the FDA's Center for Devices and Radiological Health. "
"As with other treatments for essential tremor, this new device is not a cure but could help patients enjoy a better quality of life," Peña said in a news release.
A great deal of promise has been placed in focused ultrasound technology in recent years. The technology promises to do everything from removing tumors to treating chronic pain, all through noninvasively ablating tissue.
In a double-blind control trial involving 76 patients--with 56 receiving the device and 20 receiving a fake treatment--those treated with the ExAblate Neuro showed a 50% improvement in their tremors and motor function three months after the treatment, according to FDA.
Potential adverse events for the ExAblate Neuro are similar to those for more invasive thalamotomy surgery, and could include such things as numbness/tingling of the fingers, headache, imbalance/unsteadiness, loss of control of body movements (ataxia), or gait disturbance.
Check out this video from Insightec explaining the workings of the technology:
Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
[Image courtesy of Insightec]
About the Author(s)
You May Also Like


