C.R. Bard offers a peek at more than 30 forthcoming products across its businesses, including the peripheral vascular, surgical, and urology divisions.
May 24, 2016

C.R. Bard offers a peek at more than 30 forthcoming products across its businesses, including the peripheral vascular, surgical specialties, and urology divisions.
There's plenty more where that came from.
C. R. Bard has over 150 product ideas in its pipeline but allowed analysts just a taste with a look at approximately 35 new products anticipated in the next few years. The products, which range from tools to help reduce radiation exposure from fluoroscopy to a new dialysis catheter, were described during the company's May 23 analyst meeting.
There are over 150 product ideas in the pre-R&D pipeline and "the pipeline is as robust and healthy as it's even been since I've been with the company," Tim Ring, C.R. Bard chairman and CEO, said during the meeting. The management team did not put all its cards on the table, though. "For competitive reasons, as you can imagine, some of our more exciting projects we won't talk about until we get much closer to launch," Ring added.
Get inspired to innovate in medtech at the MD&M East Conference, June 14-16, in New York City. |
Vascular
The company's Vascular business made up more than a quarter of total sales in 2015, and there are several new products anticipated in the next fews years. Several launches are planned for the Lutonix drug-coated balloon franchise, a major part of this division, including long lengths in the second half of 2016, in-stent restenosis in the first half of 2017, arteriovenous in the second half of 2017, and below-the-knee in 2019.
Also in Vascular, Bard is planning launches of it GeoAlign Marking System, to address geographic miss and to properly align therapies. This could have the added benefit of reducing fluoroscopy-based radiation exposure. "With all the benefit of fluroscopy-based, less-invasive procedures in the last 30 years, the amount of radiation our customers are exposed to has increase six-fold . . .[GeoAlign is] also able to reduce the amount of fluorscopy needed to align therapy at the treatment site, thereby reducing radiation exposure to the patient, physician, and the cath lab staff," Jim Beasley, group president of the Peripheral Vascular division, told analysts.
Other key products include the UltraScore PTA Balloon, an over-the-wire scoring balloon with the ability to dilate heavily calcified portions of vessels with less pressure. Beasley compared its capability to cutting glass: "If you score the glass with the right tool, the glass will break cleanly with very little pressure."
The LifeStream Balloon Expandable Vascular Covered Stent is expected to offer better patency versus bare metal stents when used for iliac artery disease. The product was commercialized in Europe in 2014 and earned 40% market share rapidly, Beasley noted.
Bard's Covera Covered Stent to help reduce failed arteriovenous fistulas in dialysis patients was launched in Europe in late 2015. The company expects to start enrolling patients in a U.S. IDE trial in the next few months. A U.S. study for the Venovo Stent, intended specifically for the venous anatomy in treating venous thromboembolism--the most common cause of preventable U.S. hospital deaths--is expected to begin in the next several months as well. Venovo was also launched in Europe in late 2015.
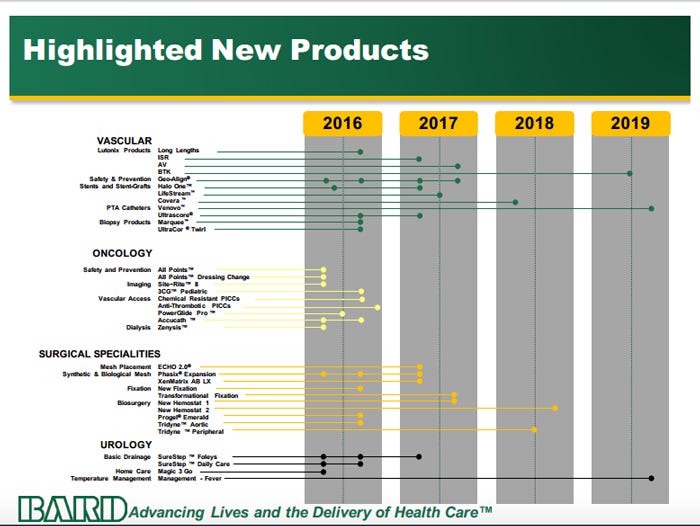
C.R. Bard's extensive product pipeline, with expected launch timelines, is shown here.
Oncology
In the Oncology division, new catheters are expected in the near future. The PowerGlide Pro midline catheter, an update on the original PowerGlide to offer improved ergonomics and ease of use, is at FDA and is expected to launch soon. A new version of the AccuCath Peripheral IV catheter launched last month, and the Zenysis Acute Dialysis catheter with 11 Fr dual-lumen catheter was released earlier this year.
Surgical Specialties
Within the Surgical Specialties business, the Echo 2 Position System for more efficient laparoscopic and robotic surgery is anticipated in early 2017. Later this year, four new sizes of the XenMatrix AB Surgical Graft are slated while 20 new sizes of the company's Phasix Mesh are expected in the third quarter.
In the next several months, two fixation systems, the OptiFix Absorbable Fixation System and the CapSure Permanent Fixation System are expected to come to market.
In addition, a few new biosurgery products, including the Progel Emerald sealant, are expected to be introduced over the next several quarters.
Urology
Tim Collins, group president of Bard's Urology business, told analysts that the company would add expanded configurations of its SureStep Tray in coming quarters. SureStep is used to simplify the numerous steps necessary for aseptic insertion of Foley catheters. Within homecare, the Magic3 Go Female Hydrophilic Intermittent catheter was recently launched.
Bard is also planning to tackle neurogenic fever prevention in stroke patients with its Arctic Sun Temperature Management System. The company is funding a large randomized clinical trial of the technology to determine whether it can prevent neurogenic fever and improve functional outcomes for these patients. The company's product timeline points to a 2019 launch.
Besides these launches, John Weiland, Bard's president and chief operating officer, reminded analysts that international launches of proven products will continue. "We also have an international R&D pipeline that comes from products that we have already proven commercially and launched in the United States and other geographies . . . As we get subsequent generations of products approved in other geographies, we have the data and the evidence to support these products for successful adoption," he said.
This volume of new products is not unusual. John DeFord, PhD, Bard's senior vice president of science, technology, and clinical affairs, said the team usually generates about 400 new ideas for products every year and 10-15% reach the development process.
In a May 23 research note recapping Bard's analyst meeting, Stifel analyst Rick Wise wrote, "Many of the products seemingly represent large potential opportunities with more than 25 projects with more than +$50M potential and roughly 10 with +$100M potential. . . As well, the company has many additional, incremental 2016-2020 product launches across each segment which we believe could help the company maintain a stable, above-market organic-growth trajectory."
[Image courtesy of STUART MILES/FREEDIGITALPHOTOS.NET.]
About the Author(s)
You May Also Like


