More than a year after it received FDA approval, the fourth generation of the Da Vinci surgical robot is seeing strong interest from customers. Now the company just needs to improve its margin on the product.
April 22, 2015
Jamie Hartford
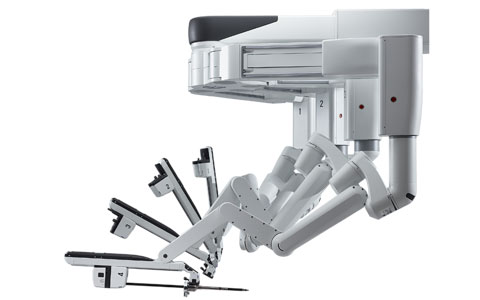
The da Vinci Xi surgical robot received FDA approval in April 2014. |
Following an abysmal 2013 that saw the company plagued by adverse event reports and a tumbling stock price, Intuitive Surgical was hopeful that last year’s launch of the da Vinci Xi System, the fourth generation of its surgical robot, would be a hit. Now, more than a year after the system received FDA approval, there are signs that could be the case.
The company exceeded Wall Street’s expectations and its own year-over-year performance by placing 99 da Vinci Systems in the first quarter of 2015—75 of them Xi models.
“da Vinci Xi continues to draw significant interest from our customers,” Gary Guthart, president and CEO of Intuitive Surgical, told analysts in an April 21 earnings call, according to a Seeking Alpha transcript.
In the United States, system placement was up 45% from the same quarter in 2014. Outside the United States, placements were down, but part of that drop could be due to a significant decline in Japan, where only one system was placed (vs. 19 in Q4 2014) as customers awaited approval of the Xi system there. Approval for the system in Japan was granted in late March.
“We continue to believe that the Xi configuration, which received FDA approval in 2Q14, will open the door to new surgical techniques and procedure growth for general surgery, especially [outside of the United States] procedures,” Sterne Agee senior research analyst Gregory P. Chodaczek wrote in a research note.
RBC Capital Markets analyst Glenn Novarro was also pleased with the Xi’s performance.
“The Xi launch is progressing nicely, which should be further aided by upcoming movable table and Xi single-site approvals,” he wrote in a research note.
Still, Chodaczek does not expect sales to skyrocket immediately.
"In line with our thesis, we expect to see continued early adopters of the Xi through FY2015 but do not expect to see considerable [quarter-over-quarter] growth in system sales of the Xi model," he wrote.
And there is room for improvement when it comes to costs associated with the Xi system. Intuitive Surgical’s margins took a hit last quarter in part due to higher manufacturing costs as a percentage of revenue associated with the system.
“New products like Xi and stapling have lower gross margins earlier in their life cycle than our mature products,” said Marshall Mohr, Intuitive Surgical’s chief financial officer. “We believe our efforts to reduce the cost of these products will begin to deliver limited improvements in our gross margins by the end of this year and greater improvement in fiscal 2016.”
But Guthart cautioned that Xi’s margins may never reach those of its predecessor, the da Vinci Si.
“We have always provided the caveat that it doesn’t necessarily mean you’ll get to the same level as our mature products,” he said. “They are highly complex products . . . And so we never committed that we would get back there. Having said that, I think that they had room to improve from where we are and we are working hard on those improvements.”
The Xi system is intended to replace open abdominal surgeries and includes several advances over the Si model, including an overhead instrument arm architecture; an improved endoscope digital architecture; smaller, thinner arms that provide a greater range of motion; and longer instrument shafts.
Learn more about designing innovative medical devices at the MD&M East conference in New York City June 9–11, 2015. |
Jamie Hartford is MD+DI's editor-in-chief. Reach her at [email protected] or on Twitter @MedTechJamie.
[image courtesy of INTUITIVE SURGICAL]
You May Also Like


