March 31, 2017
Owlstone Medical will use new funding to commercialize its early-detection technology.
Nancy Crotti
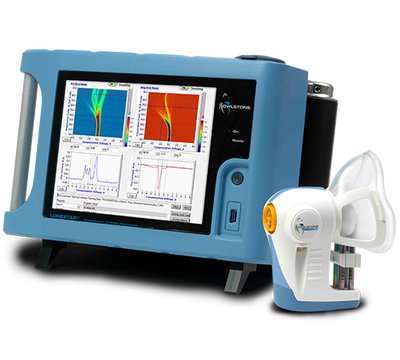
Owlstone Medical's FAIMS platform is designed to detect disease biomarkers in breath.
A British company developing "breath biopsy" technology to detect early-stage cancer has landed an investment from a major insurer.
The undisclosed investment from Aviva Ventures brings total funding for Owlstone Medical to $23.5 million. Aviva Ventures is the venture capital arm of London-based Aviva plc, the largest general insurer in the United Kingdom.
The money will help Cambridge, England-based Owlstone to accelerate the commercialization of its technology and support its clinical trials. Owlstone recently launched a 1,400-patient breath-based study using its microchip Field Asymmetric Ion Mobility Spectrometer (FAIMS) technology to detect bowel cancer.
The InTERCEPT trial is designed to evaluate the accuracy of Owlstone's test in the detection of early-stage colorectal cancer when patient survival rates are at their highest. The company is also involved in the LuCID 3,000 patient trial for early detection of lung cancer. These trials are the largest clinical trials ever undertaken for early detection of cancer in breath.
A pilot study using the technology showed a sensitivity of 88% in detecting volatile organic compound biomarkers for colorectal cancer. The pilot study also showed a sensitivity of 62% for detection of advanced adenomas, a precancerous stage of colorectal cancer, representing a substantial increase in the rate of detection compared with the fecal occult blood tests based on detecting blood in feces that are used currently within the NHS bowel cancer screening program.
The company's breath collection device, the ReCIVA Breath Sampler, has the CE mark and is in use in more than 60 academic research groups and clinical labs around the world. Its quality assurance and regulatory affairs managers have been in touch with FDA regarding U.S. approval.
The ability to diagnose disease by taking a breath biopsy will save lives and cut treatment costs, according to Owlstone founder and CEO, Billy Boyle. Boyle lost his wife to late-diagnosed colorectal cancer.
"Early detection is the greatest opportunity that we have to improve outcomes for patients," Boyle told Qmed in an email. "The added benefit of detecting disease earlier is that it becomes easier to treat, and the cost of treatment for healthcare providers is reduced, potentially leading also toreduced insurance premiums."
Aviva will get an undisclosed ownership state in Owlstone and will seek to actively promote
Owlstone Medical and its breath biopsy technology, according to Boyle. Other investors include international entrepreneur network Medtekwiz, and a number of U.S. based VCs.
"We expect that association of Owlstone Medical with the Aviva brand will raise awareness of breath as a new diagnostic modality within the healthcare space," he said. "This will be extremely helpful to drive adoption of the breath biopsy technology as we move forward."
Nancy Crotti is a contributor to Qmed.
[Image credit: Owlstone Medical]
About the Author(s)
You May Also Like


