S-ICD System
September 28, 2016
1 Min Read
S-ICD System
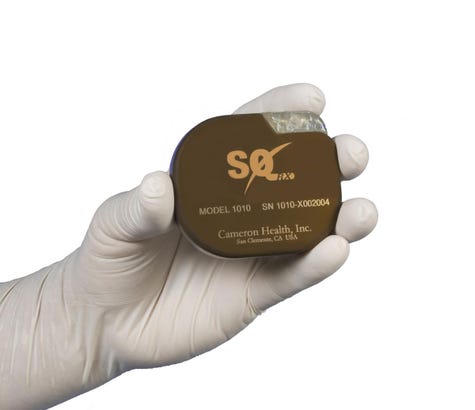
Boston Scientific's S-ICD System offers many patients cardiac defibrillation without transvenous leads. Leads can come with complications, such as migration and perforation, and attempts to remove leads can be risky too. The S-ICD is implanted subcutaneously and does not contact the heart or veins. In August 2016, FDA granted approval to the third-generation Emblem MRI S-ICD, an MR-conditional device. The S-ICD System won the Prix Galien "Best Medical Technology" award in 2013. |
|
[Image courtesy of BOSTON SCIENTIFIC CORP.]
Learn about "How to Set Your Connected Health Solution Apart" at BIOMEDevice San Jose, December 7-8. |
Sign up for the QMED & MD+DI Daily newsletter.
You May Also Like




