BrainScope
September 8, 2016
BrainScope
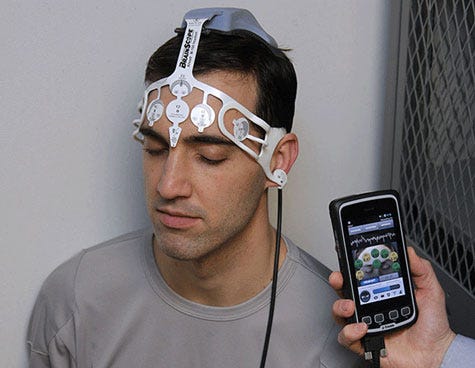
BrainScope, headquartered in Bethesda, MD, is working to find a way to determine which patients have concussions. BrainScope's Ahead 200 device (pictured above) received FDA 510(k) clearance in May 2015. The device takes an electroencephalograph (EEG) reading of a patient with mild TBI. With a smartphone and a custom sensor, the handheld system is used to assess damage to the brain. According to the corporate website, the Ahead 200, as well as its predecessor, the Ahead 100, are meant for development, not commercial, purposes. Its next-gen system is in the works. The company was also one of the final winners in the Head Health Challenge I, sponsored by NFL and GE. Its TBI assessment technology was highlighted at the NFL Annual Meeting in March 2016. BrainScope has also received millions in TBI research funding from the U.S. Department of Defense. |
|
[Image courtesy of BRAINSCOPE]
Learn about "Tapping the Explosion of Offerings in Sensors" at the MD&M Minneapolis Conference, September 21-22. |
You May Also Like




