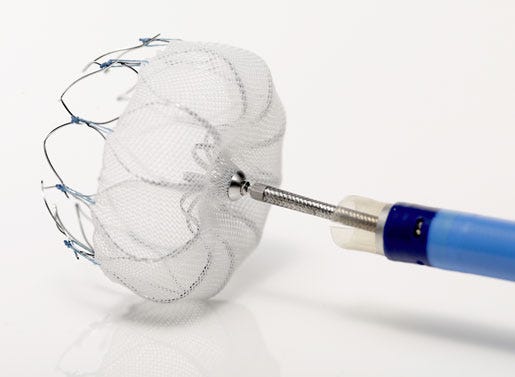
Boston ScientificBoston Scientific got positive reimbursement news for its Watchman left atrial appendage closure device in 2016.
October 19, 2016
Boston Scientific |
Boston Scientific got positive reimbursement news for its Watchman left atrial appendage closure device in 2016. |
In early February 2016, the rollercoaster that Boston Scientific's Watchman left atrial appendage closure device took to market finally came to rest when CMS backed off a 2015 proposal to limit reimbursement coverage to patients who can't take the anticoagulant drug warfarin. The decision was lauded by company management, which predicted the device could open up a $500 million market opportunity worldwide. Following that win, Boston Scientific saw a steady stream of FDA approvals, for its Acuity X4 quadripolar left ventricular leads--one of the most hotly anticipated devices of the year--later in February, for its ImageReady MR-conditional pacing system in April, and for its MR-conditional Emblem S-ICD in August. This year has also brought strong sales for the comapny's Synergy bioabsorbable polymer drug-eluting stent, a continuation of its collaboration with Mayo Clinic, and the acquisition of EndoChoice Holdings, which analysts said could help keep the company's endoscopy business booming. Add to all that building buzz about the still-in-development Empower leadless pacemaker, CE Mark for the Lotus Edge next-gen transcatheter aortic valve, and a restructuring effort intended to free up to $150 million for "strategic growth initiatives" by 2020, and the future continues to look bright for Boston Scientific. |
[image courtesy of BOSTON SCIENTIFIC] |
You May Also Like