Endologix's Nellix
January 22, 2016
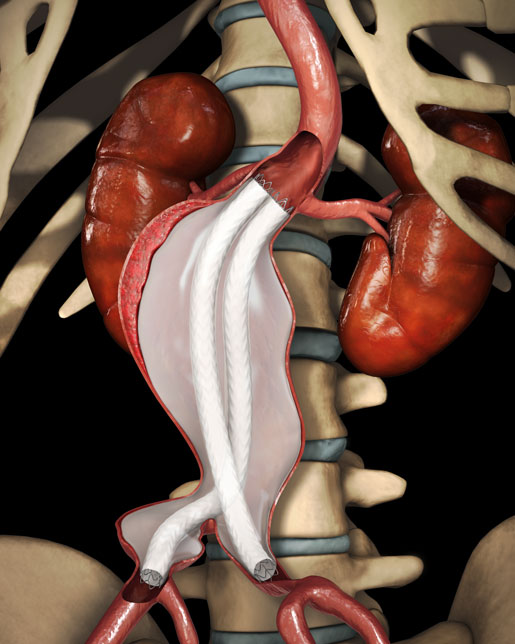
Endologix's Nellix EndoVascular Aneurysm Sealing (EVAS) system is intended for treatment of patients with abdominal aortic aneurysms (AAA) located below the renal arteries. The device is different from other endovascular AAA repair systems because it is designed to seal the aneurysm sac. According to the corporate website, Nellix may offer ease-of-use, ability to be used in a wider range of patient anatomies, and the possibility of fewer reinterventions to treak endoleaks or device migration. Nellix received CE Mark in January 2013. Endologix plans to complete PMA submission in early 2016 and expects FDA approval by end of 2016. These products do not have U.S. FDA regulatory clearance or approval as of January 22, 2016. |
|
[Image courtesy of ENDOLOGIX, INC.]
Check out the future of medical technology at the world's largest medical design and manufacturing event—register for the MD&M West Conference, February 9-11, 2016. |
You May Also Like




