Abbott's Absorb GT1
January 22, 2016
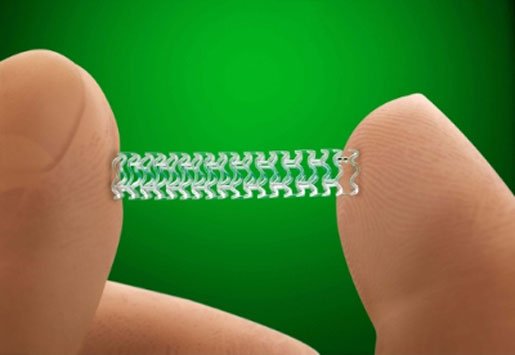
Abbott's Absorb GT1 Bioresorbable Vascular Scaffold (BVS) is a drug-eluting device that is used in angioplasty to open a narrowed coronary artery. The scaffold eventually dissolves, so patients no longer have a foreign device in their arteries. Absorb received CE Mark back in January 2011, making it the first drug-eluting BVS to be approved. The Absorb GT1 with the GlideTrack catheter received CE Mark in May 2015. Competitor Boston Scientific's Synergy received FDA approval in October 2015 and is the first U.S. drug-eluting stent system with a bioabsorbable polymer and drug coating. Last year, Abbott filed a U.S. submission for FDA approval of the Absorb GT1 BVS. The product's PMA application will be discussed and voted on during a March meeting of the Circulatory System Devices panel. Timing is never certain and FDA is under no obligation to heed the panel's view, but this could lead to a regulatory decision in 2016. These products do not have U.S. FDA regulatory clearance or approval as of January 22, 2016. |
|
[Image courtesy of ABBOTT]
Check out the future of medical technology at the world's largest medical design and manufacturing event—register for the MD&M West Conference, February 9-11, 2016. |
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)

