July 27, 2015
1 Min Read
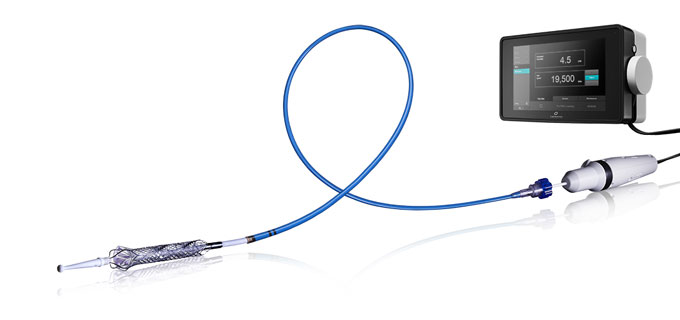
Thoratec's HeartMate PHP
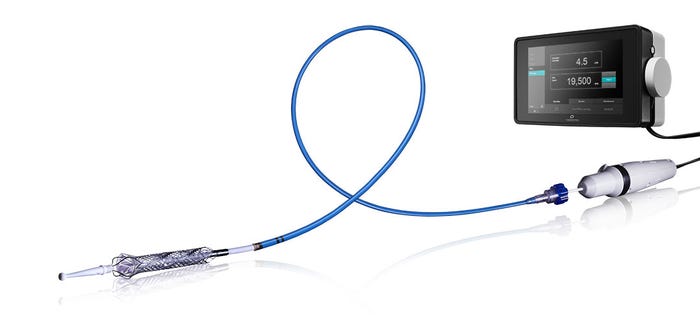
Thoratec's HeartMate PHP is a heart pump intended for hemodynamic support in high-risk percutaneous coronary intervention (PCI) patients. HeartMate PHP received CE Mark in July 2015 but does not yet have FDA approval. The SHIELD II U.S. pivotal trial is to be conducted in patients undergoing high-risk PCI to support an FDA approval. The randomized trial pits HeartMate PHP against Abiomed's Impella 2.5, which has FDA approval and is the only direct competitor. Analysts expect HeartMate PHP could receive FDA approval in 2018. |
|
[Image courtesy of THORATEC CORP.]
Sign up for the QMED & MD+DI Daily newsletter.
You May Also Like




