July 27, 2015
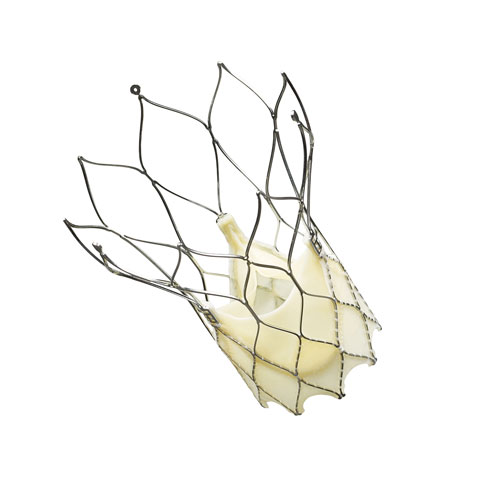
St. Jude Medical's Portico
St. Jude Medical's Portico transcatheter aortic valve is a repositionable, retrievable implant that has had CE Mark since . Portico received CE Mark for its 23 mm size in November 2012 and for its 25 mm size in December 2013. The valve is being studied in a U.S. pivotal trial for FDA approval. In September 2014, both commercial and trial implants were halted to investigate what was thought to be unusual reduced mobility of the valve's leaflets. However, CE Mark selling was allowed again in March 2015 and the U.S. trial was cleared to restart in June 2015 after it was found this reduced leaflet mobility is seen across all transcatheter and surgical aortic valves. Executives recently said they expect larger sizes of Portico to receive CE Mark in the second half of 2015 and that U.S. trial patients should start receiving implants again later this quarter (July–September). |
|
[Image courtesy of ST. JUDE MEDICAL, INC.]
You May Also Like



