July 27, 2015
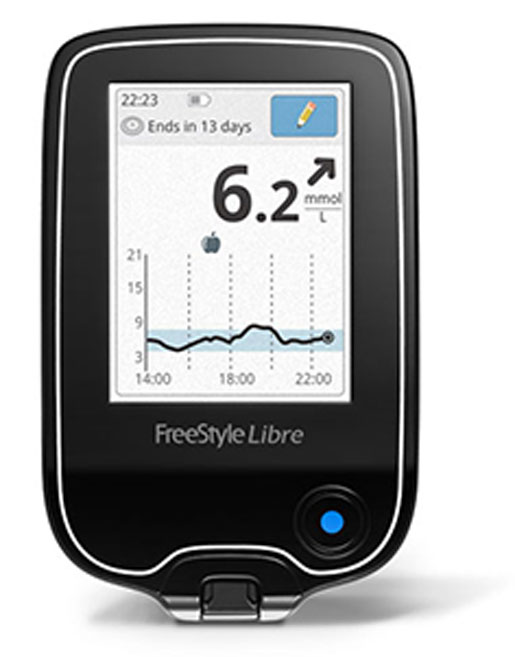
Abbott's FreeStyle Libre Flash Glucose Monitoring System
Abbott's FreeStyle Libre Flash Glucose Monitoring System allows patients to measure their blood glucose levels without fingerpricks or test strips. The system consists of a small sensor that is worn on the back of the arm for as long as 14 days and a reader. The sensor has what the company describes as a "small filament" that is inserted into the arm and measures blood glucose levels in the interstitial fluid. A reader is passed over the sensor to give the patient a real-time glucose reading in less than a second. The Libre Flash received CE Mark in September 2014 and on a recent earnings call, executives described it as "a real winner of a product," noting that the company does not yet have enough capacity to keep up with demand. Abbott has filed a regulatory submission with FDA for approval of a professional version, Libre Pro, for use in the United States. |
|
[Image courtesy of ABBOTT]
You May Also Like




