July 24, 2017
Boston Scientific led a $35.5 million funding round to support Amphora Medical's bladder denervation study.
Kristopher Sturgis
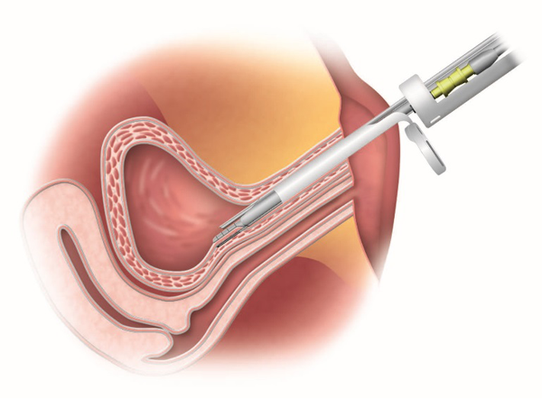
Amphora Medical said its recent $35.5 million funding round will support a U.S. study of Selective Bladder Denervation System for women suffering from an overactive bladder.
A company looking to bring its Selective Bladder Denervation System to the U.S. market for women with overactive bladders has raised $35.5 million in a round led by Boston Scientific. Longitude Capital and HBM Healthcare Investments also participated in Amphora Medical's recent financing.
Boston Scientific's investment in Amphora could indicate that the company is taking a stronger interest in the bladder device market. Two years ago the company acquired Endo International's American Medical Systems urology portfolio for $1.6 billion.
Amphora's technology is designed to produce targeted ablation to reduce nerve signals in the bladder that are responsible for many of the symptoms associated with an overactive bladder. The cystoscopic device also works to preserve the nerves responsible for natural bladder function, allowing the system to provide lasting symptom relief to patients through a simple-to-use, intuitive device, according to the company.
The announcement comes as a strong signal of intent by the Minnesota startup, which is targeting a market currently led by Medtronic. But Amphora is not the only company looking to bring new treatment options to patients with OAB. Axonics recently raised $35 million, most of which has been slated to fund its rechargeable sacral neuromodulation system for patients with urinary and bowel dysfunction.
Medtronic also offers a sacral neuromodulation device, the Interstim System, but it is not rechargeable. The system is designed to stimulate the sacral nerve -- the nerve responsible for relaying a variety of sensations, like the fullness in the bladder or rectum, to the brain.
Instead of stimulating the sacral nerve, Amphora's bladder denervation system is designed to dampen specific nerve signals that are often responsible for OAB symptoms.
Amphora CEO Tom Ressemann said the latest funding round will help the company move the technology into the next phase of testing as it prepares to enter the U.S. market.
"The closing of this round is an important milestone for Amphora as we work to advance our minimally-invasive Selective Bladder Denervation System as a treatment for OAB," Ressemann said. "Upon the completion of two ongoing feasibility studies, we expect to initiate the pivotal, randomized, sham-controlled, selective bladder denervation clinical trial in women who are refractory to medical therapy in 2018."
Kristopher Sturgis is a contributor to Qmed.
[Image credit: Amphora Medical Inc.]
About the Author(s)
You May Also Like


