Getting medical device manufacturing designers, engineers, and production teams synchronized on a common product model improves product quality by reducing errors in production and increasing yield rates.
Medical device manufacturers face hurdles taller than ever in getting high-quality products to market faster, cost-effectively. Hindering their efforts are not just external factors but also internal processes for designing and producing those products. That is why it’s time for medical device companies to adopt a design-to-manufacturing environment where designers, engineering, and production teams are synchronized on a common product model. This approach improves both product quality and profitability by reducing errors in production while increasing yield rates.
Let’s look at the challenges medical device manufacturers face in bringing products to market today, the principles behind design-to-manufacturing, and how this approach can help companies to transform their business.
The Challenges of Creating High-Quality, Innovative New Products
Design, engineering, and research and development (R&D) costs for creating a new medical devices are among the highest in manufacturing today. According to a 2010 study completed by Stanford University, FDA’s Impact on U.S. Medical Technology Innovation, it costs $31 million to develop a medical device from concept to market. Pre-Market Approvals (PMA) cost medical device manufacturers on average $94 million per device, and $75 million of the PMA is for FDA-based compliance alone.
At the same time, a proliferation of new products reaching PMA levels puts pressure on medical device manufacturers to achieve product and feature parity from a product roadmap perspective with their competitors. According to a 2019 Drugwatch article, FDA’s Center for Devices and Radiological Health (CDRH) reports that they receive about 22,000 submissions for clearance or approvals of new medical devices every year.
Adding to the competitive intensity is the strategy many medical device manufacturers have of introducing low-end models that compete on price. Accepting all of these challenges while complying with regulatory and industry reporting and quality standards makes medical device manufacturing one of the most capital-intensive industries there are.
Given how tight the new product development timelines are and how expensive each product is to produce, designers, engineers, and production teams need to be completely synchronized. Knowing the specifics of product models across manufacturing reduces errors in production and increases machinery yield rates that prolong the life of machinery across production centers. But too often, medical device companies’ computer-aided design (CAD), simulation/finite element analysis (FEA), electrical, computer-aided manufacturing (CAM), inspection, and work instructions that feed into the design-to-manufacturing process run at completely different cadences or clock speeds than the enterprise resource planning (ERP) systems on which manufacturing teams rely. For medical products manufacturers to achieve their time-to-market, quality, and cost goals, each of these systems needs to be integrated and synchronized within a design-to-manufacturing workflow supported at the platform level.
Creating a Design-to-Manufacturing Environment
Syncing the diverse base of manufacturing systems together creates a single design-to-manufacturing environment where designers, engineers, and production teams can collaborate together in real time. By taking a concurrent and integrated approach, engineering, quality, and manufacturing teams have the ability to share data on existing and new products under development, and fewer errors are made in defining how they will be produced. This change, alone, extends the life of machines on the shop floor, enabling them to produce consistently higher quality products.
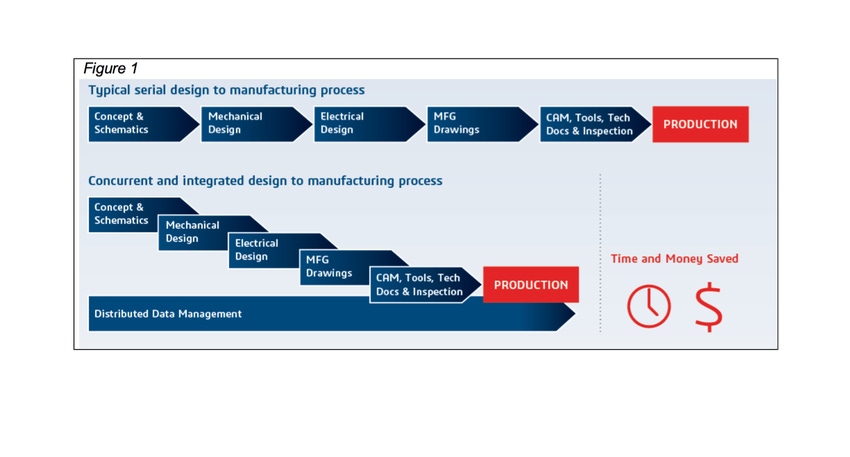
Figure 1 (at top) contrasts the typical serial approach versus a concurrent and integrated approach to the design-to-manufacturing process. Notably, the latter approach can save medical device companies roughly 25% in time and money.
In moving to concurrent and integrated design-to-manufacturing process, engineering, quality, and manufacturing teams need to take more of a lifecycle-based view of each product, relying on their CAD systems’ representation of product models as a single source of the product definition.
When designers, engineers, and manufacturing teams aren’t using the same product definitions, product quality drops fast. Production machines and the teams running them don’t receive accurate work instructions, and suppliers send components and materials that don’t match the product design.
When there is an accurate, multifaceted definition of every product model in the CAD systems, product models can serve as the single source of a product definition, and all changes to the product at the bill of material (BOM) level can be propagated automatically through all functional areas. Concurrent design and manufacturing are possible at a significantly faster pace, since there’s no need to freeze designs to include any product changes.
Increasing Quality and Innovation While Cutting Costs
Medical device companies that adopt a concurrent and integrated design-to-manufacturing process—where their CAD, simulation/FEA, electrical, CAM, inspection, quality management, work instructions, ERP, and manufacturing execution system (MES) software are synchronized, with CAD systems’ product definitions serving as the central product definition—can increase their quality and innovation while cutting costs in three key ways.
Speed time-to-prototype. Syncing the diverse base of systems enables medical device manufacturers to reduce the time-to-prototype exponentially while increasing product quality. When centralized product models managed in CAD systems are the main product definition engineering and manufacturing rely on, teams have the analytics, data, and information they need to take action, in the language or lexicon they speak. As a result, companies adopting this approach are seeing a proliferation of new product prototypes with fewer initial design and prototype errors while also protecting against and future production issues.
Foster collaboration. Medical device manufacturers who adopt a design-to-manufacturing approach to managing the lifecycles of their products free up engineers and production teams to work interactively and solve manufacturing challenges faster. Manufacturing product engineers can use design-to-manufacturing environments or platforms to evaluate new product designs earlier in the product development process. And manufacturing scheduling teams can look at the impact of new models on existing shop floor workflows as well as wear and tear on new machines. This results in the ability to both increase manufacturability and reduce costs.
Pursue configure-price-quote selling. The total available market for medical devices increases the more a given medical device manufacturer can offer customers and distributors flexibility in product designs. Taking a lifecycle-based approach where the centralized product model serves as the single product definition company-wide can free medical product manufacturers up to pursue a configure-price-quote (CPQ) and product configuration strategy. This, in turn, can deliver higher gross margins by attracting customers who otherwise would not have purchased devices.
Conclusion
Taking a centralized product model approach that scales across the entire design-to-manufacturing process combined with a collaborative working environment helps to increase the levels of innovation medical products manufacturers can achieve. A concurrent and integrated design-to-manufacturing process makes it possible for medical device manufacturers to deliver products faster, at a higher overall level of quality, and at lower costs. It’s time for medical device manufacturing to adopt a more lifecycle-based approach to creating new products, one that brings together design, engineering, and manufacturing interactively on a real-time collaborative platform.
About the Author(s)
You May Also Like




