Enrollment for the CardiAmp Cell Therapy System could be completed by the end of 3Q19.
November 16, 2018
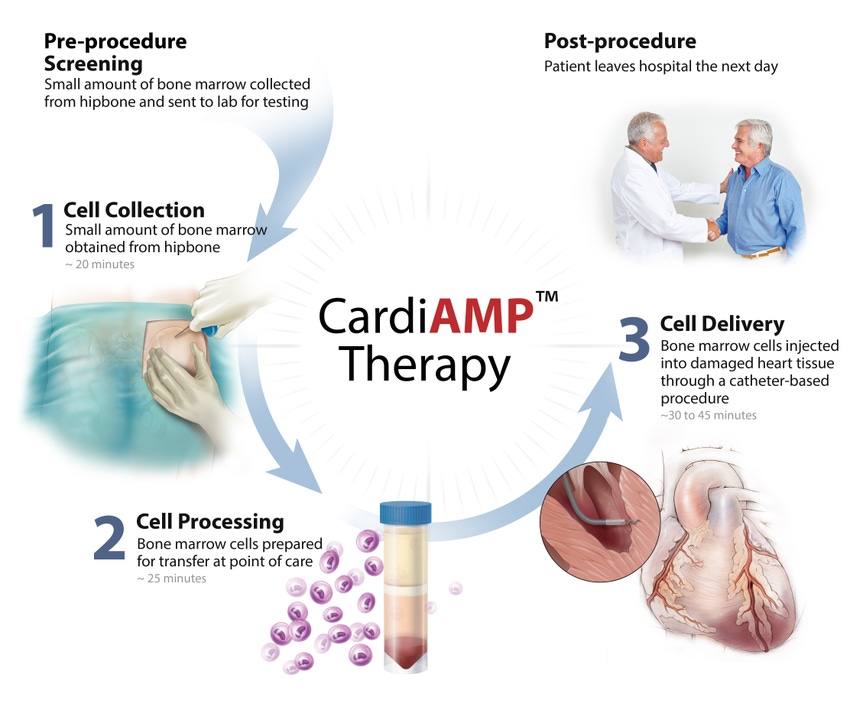
It can be a bit tricky when trying to put BioCardia’s cell therapy for heart failure in one specific category. Is the CardiAmp Cell Therapy a diagnostic, device, or a biologic? Perhaps it’s all of the above. The San Carlos, CA-based company’s president and CEO painted a clearer picture for the technology noting that it could play a significant role when looking at heart failure treatment.
“The [CardiAMP Cell Therapy System] is regulated by the agency [FDA] as a combination product via an IDE PMA device pathway,” Peter Altman, BioCardia’s CEO, told MD+DI. “But it’s regulated by the Center of Drug Evaluation and Research [CEDAR].”
He added, “The technology we’re advancing … is a comprehensive approach to autologous cell therapy. It’s comprehensive in both our application of diagnostics to really select patients most responsive for therapy in the trial; the cell processing technology; and the device delivery technology.”
CardiAMP cell therapy uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally-invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. The procedure takes under an hour.
BioCardia has begun enrollment for a pivotal trial of the CardiAmp Heart Failure system. The trial could enroll up to 260 patients.
“Right now, we’re still focusing on the data and the trial enrollment,” Altman said. “Currently we project completing enrollment at the end of 3Q19.”
The company recently reported 12-month data at the American Heart Association Scientific Sessions 2018, for the 10-patient roll-in cohort of the pivotal CardiAMP Heart Failure Trial studying adult patients experiencing heart failure following a heart attack.
Results show at the primary endpoint of exercise capacity at 12 months, the 10-patient roll-in cohort of the trial showed clinically meaningful improvement, walking an average of 46.4 meters more than baseline (N=10), although the improvement was not considered statistically significant (p=0.063). Eight of the 10 patients experienced improvement in their exercise capacity.
This improvement is more than triple the average improvement over baseline reported in the CardiAMP-treated arm of the Phase II TAC-HFT-MNC trial, and greater than the average improvement seen in a number of pivotal trials for implantable cardiac resynchronization therapy (CRT) to treat heart failure.
In the secondary efficacy endpoint of quality of life, patients showed a clinically meaningful improvement of 9.8 points relative to baseline, which was not statistically significant (p=0.331) in the small cohort. Seven of the 10 patients reported better quality of life after CardiAMP treatment. This was a greater improvement over baseline than was seen in the Phase II TAC-HFT-MNC trial of CardiAMP therapy.
“Beta blockers have shown they can both enhance heart function and slow deterioration in heart failure patients; however, many patients are already on them and still do not have the level of improvement that truly improves their quality of life,” Altman said. “When you look at interventions, here’s what I find really compelling about what we’re doing – neither the standard of care, which is cardiac resynchronization therapy, nor any other cell therapy, has shown that they can actually improve heart function or symptoms. We have been able to show in both our Phase II trial and now in early results from our Phase III/pivotal trial that patients improve in clinically meaningful ways from their baseline, and not simply against a placebo.”
About the Author(s)
You May Also Like




