Automation
Samsung advanced materials lab
Components
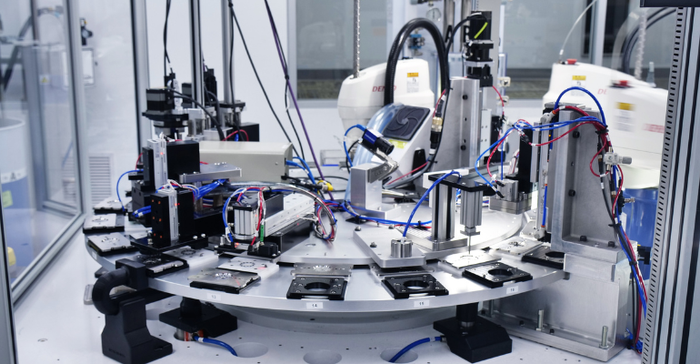
New Approach, Automated Lab Streamlines Battery Chemistry TestingNew Approach, Automated Lab Streamlines Battery Chemistry Testing
University of Michigan research showcases how automated laboratories expedite chemical testing, enabling transformative battery advancements.
Sign up for the QMED & MD+DI Daily newsletter.
.png?width=700&auto=webp&quality=80&disable=upscale)