Medical Device & Diagnostic Industry MagazineMDDI Article IndexINTO THE INTERNETOriginally Published June 2000MEDICAL PLASTICS AND BIOMATERIALS:For medical device developers and manufacturers, adhesives offer numerous benefits that cannot be obtained using traditional welding techniques.Christine Salerni
June 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
INTO THE INTERNET
Originally Published June 2000
Christine Salerni
Adhesives have long played an integral part in the assembly of components for a wide variety of markets, including the medical market. Anaerobic sealants, cyanoacrylates, light-curable acrylics, and silicones are used in diverse lifesaving applications ranging from automotive air-bag sensors to catheters and blood oxygenators. Each industry has unique requirements for adhesives—from appearance and bond performance to environmental resistance.
Over the past two decades, the medical disposables market has undergone a number of changes in the types of devices produced, substrates selected, and sterilization procedures employed. In the early 1970s, manufacturers were using materials like glass, rubber, and metal to assemble syringes, surgical instruments, and other devices. Products made from such materials were typically machined, molded, or assembled with fasteners. As advances in technology in the 1980s resulted in intricate, high-performance device designs, the need for engineering plastics became apparent. During this same period, a pronounced shift to single-use devices forced design engineers to evaluate such polymers as acrylic, polycarbonate, and PVC.
TYPES OF DEVICES
Traditionally, adhesives manufacturers have focused on two types of medical devices: sterile disposables and nonsterile reusables. Sterile disposables are devices such as syringes, catheters, and oxygenators, which may come into contact with blood or bodily fluids. Because of such contact, the adhesives used in sterile devices must be thoroughly tested by the manufacturer and must pass stringent toxicity testing.
 The splitable stainless-steel needle for this microcatheter is bonded to the lightly tinted polycarbonate wings with a light-cure adhesive.
The splitable stainless-steel needle for this microcatheter is bonded to the lightly tinted polycarbonate wings with a light-cure adhesive.
Nonsterile reusables are generally external devices that do not come into direct contact with patients. Typically, materials conducive to long-term use are selected for the construction of such devices, and the adhesives used in their assembly do not require toxicity testing or resistance to sterilization.
In recent years, two additional classes of medical devices have emerged: sterile reusables and resposables. Sterile reusable devices—which have undergone tremendous market growth recently due to advances in less-invasive surgery—include surgical equipment such as endoscopes and laparoscopes. Resposables, on the other hand, are devices that were initially intended for one-time use but are now being considered for reuse. In any case, whether the devices undergo single or multiple sterilization cycles or whether the materials selected are the traditional glass and metal or thermoplastic resins, medical device designers will almost uniformly consider adhesives among the options for assembly.
REGULATORY ISSUES
In addition to performance issues, medical device manufacturers must also consider regulatory requirements. Device manufacturers rely on their component suppliers for assurance that neither the substrate nor the adhesive will cause problems with the biocompatibility of the device. In an effort to address such issues, both raw material suppliers and adhesive manufacturers now test their components using procedures similar to those used to qualify end-use devices.
Guidelines established by the United States Pharmacopeia (USP), which were initially employed to determine the suitability of plastics for use in medical devices, are also followed by adhesive manufacturers. Adhesives are tested for their effect on cells (cytotoxicity) and blood constituents (hemolysis), for their effect on adjacent tissues following implantation, and for their overall systemic effect. Exposure temperatures, the particular solvents used for extraction, and test duration can all vary according to the supplier and should be examined closely. Although several classes of biocompatibility exist, adhesive suppliers test to USP Class VI, indicating that the material/ device may come into contact with bodily fluids. The results of Class VI testing are typically provided to device manufacturers in the form of certificates of compliance, on an as-requested basis.
 Cyanoacrylate adhesive is used to bond flexible PVC tubing to TPE on a fistula used in dialysis.
Cyanoacrylate adhesive is used to bond flexible PVC tubing to TPE on a fistula used in dialysis.
Over the past 5 to 10 years, several countries have come together to develop a more widely accepted standard for biocompatibility testing—ISO 10993. Compared with Class VI testing, the ISO standard typically involves additional tests, extraction solutions, extraction temperatures, and test durations. ISO 10993 is recognized by diverse North American, European, and Asian countries and is quickly becoming the standard of choice for companies operating in the global arena.
A variety of questions should be asked by the end-user during the selection process for an adhesive supplier, including:
What standard is the adhesive supplier testing to?
What qualification tests in the standard does the supplier include?
How frequently are products retested to verify compliance?
How are test specimens prepared? Are bonded or coated assemblies tested?
What are the extraction conditions?
What type of documentation can the supplier provide to verify compliance?
End-users need information about the testing parameters employed as well as the manner in which test specimens are prepared in order to ensure that they understand any referenced compliance. For example, some adhesive suppliers may test an actual device, but because each device is unique in materials, joint design, and end use, a generalized claim of a "biocompatible adhesive" may be meaningless to companies producing different types of devices. It is also important for device manufacturers to recognize that adhesive biocompatibility compliance is a guideline only, and not a guarantee. Should the adhesive be improperly cured, for instance, biocompatibility may be sacrificed.
DEVICE ASSEMBLY METHODS
Device manufacturers employ a variety of assembly methods, including solvent welding, ultrasonic welding, vibration welding, and adhesive bonding. Each method offers benefits along with limitations, as outlined in Table I.
Solvent | Ultrasonic | Vibration | Adhesive |
Benefits | |||
Low Cost | Easily automated | Simple | Joins dissimilar |
Limitations | |||
Not usable on | Not usable on | Not usable on | Requires cure |
Table I. Benefits and limitations of medical device assembly methods.
The use of solvent welding for device assembly has become both a manufacturing and an environmental issue. As environmental regulations and operator safety become prime concerns for manufacturers throughout the world, the use of solvents in a production environment has become more problematic. Ozone-depleting chemicals have often been replaced by flammable, user-unfriendly alternatives, which are now causing designers to seek yet other options. In addition, solvent welding cannot be used effectively on thermoset plastics, and is more likely than other liquid-joining methods (i.e., adhesives) to cause stress cracking of components. Joints with large, induced gaps cannot be secured via solvent bonding or welding. Solvent application volume and time are also critical factors that must be closely monitored in order to ensure performance consistency.
Ultrasonic welding—the most widely used welding method—is quite often the joining method of choice for design engineers because of its simplicity of use and rapid-joining potential. The process employs ultrasonic energy at high frequencies to produce mechanical vibration, creating heat at the joint interface and a slight melting of the substrate surfaces. Such a process can take as little as 1 second depending on substrate, joint design, and equipment employed.
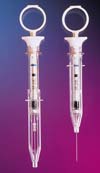 The stainless-steel cannula of this safety syringe is bonded to the K-resin syringe body using light-cure adhesives.
The stainless-steel cannula of this safety syringe is bonded to the K-resin syringe body using light-cure adhesives.
There are, however, a number of drawbacks limiting the usefulness of ultrasonic welding. Like solvent welding, ultrasonic welding cannot be used on thermoset plastics and is sensitive to bond-line configuration. Large gaps or minimal contact areas pose problems for ultrasonic welding processes. When dissimilar plastics are used, differing melting points and potential chemical incompatibility between the two materials must be considered. Ultrasonic welding also requires significant investment for capital equipment as well as special tooling for each unique assembly.
Vibration welding involves the melting of the joint of an assembly via friction caused by the vibration of two substrates in contact with each other. For such a process to work, the materials selected must be thermoplastics, must be somewhat rigid, and must have either flat or slightly curved interfaces. As with various other welding processes, significant capital expenditure and special tooling are required. In addition, vibration welding of substrates that contain high levels of moisture may result in the formation of bubbles during processing, which, in turn, may produce a poor aesthetic appearance or weak interfacial bonds. Of the two types of vibration welding, linear welding (substrates moving linearly to each other) is more common than orbital welding (upper substrate moving in a circular direction). In either case, weld flash is a common result of vibration-welded components.
 A dual-curing silicone adhesive affixes the molded tip onto this silicone wound-drain extrusion.
A dual-curing silicone adhesive affixes the molded tip onto this silicone wound-drain extrusion.
ADHESIVES FOR DEVICE ASSEMBLY
The types of adhesives commonly used for medical device assembly include cyanoacrylates, light-curable acrylics, light-curable cyanoacrylates, epoxies, urethanes, and dual UV/ moisture-curable silicones. In general, adhesives offer several benefits compared with other assembly methods, including the ability to fill large gaps, to bond dissimilar materials, to distribute stress evenly across a bond line, and to form a hermetic seal when confined between two substrates.
Cyanoacrylates. Cyanoacrylates are polar, linear molecules that undergo an anionic polymerization reaction (see Figure 1). A weak base—such as the moisture present on essentially all surfaces—triggers the reaction, causing linear chains to form. The products are maintained in liquid form through the addition of weak acids that act as stabilizers. A variety of cyanoacrylate formulations are available, with varying viscosities, cure times, strength properties, and temperature resistance.
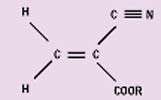 Figure 1. Typical
Figure 1. Typical
cyanoacrylate monomer.
When cured, cyanoacrylates form thermoplastic resins. The initial cyanoacrylate resins, introduced in the 1950s by Eastman Kodak, possessed several performance limitations that were rectified in later formulations. Standard, unfilled, ethyl monomer–based cyanoacrylates typically exhibit low impact and peel strengths, low to moderate solvent resistance, and maximum operating temperatures of 160°–180°F.
In the late 1970s, the addition of rubber to standard ethyl cyanoacrylate formulations resulted in significant improvements in peel and impact strengths. A standard ethyl cyanoacrylate tested in peel mode provides an average strength of less than 3 lb per width inch (pwi). In comparison, a rubber-modified cyanoacrylate exhibits peel strength of approximately 40 pwi. The addition of compounded rubber to the ethyl formulations does have a slight effect on fixture time: whereas a typical fixture time for a standard ethyl can be as low as 3 seconds on select substrates, a rubber-toughened ethyl requires between 30 seconds and 2 minutes to develop fixture strength.
Another potential drawback of cyanoacrylate adhesives is blooming or frosting—the presence of a white haze around the bond line. This phenomenon is caused by the reaction of volatilized cyanoacrylate monomer, which, because it is heavier than air, actually falls back to the surface and settles around the bond. Certain cyanoacrylate adhesives now use monomers with a higher molecular weight and lower vapor pressure, thus minimizing the potential for blooming. Users of these types of products should be cautioned, however, since the change in monomer does have an effect on cure speed, physical properties, and operating temperatures.
Traditional ethyl- or methyl-based cyanoacrylates can typically withstand maximum temperatures of approximately 180°F. Recent advancements in ethyl cyanoacrylate technology now allow thermally resistant products to withstand continuous-exposure test temperatures up to 250°F. Such thermally resistant adhesives are typically modified, toughened cyanoacrylates and therefore feature decreased fixture times. Toughened cyanoacrylate formulations are available in both black and clear versions.
Besides advancements in ethyl cyanoacrylate technology, there have also been significant developments in primer and accelerator formulations that not only offer speed of cure but also the ability to join hard-to-bond plastics. The primers are solvent-based systems that deposit reactive species onto otherwise "dead" substrates. Such reactive species allow for significant increases in bond strength of many materials that are normally difficult to bond, including polyethylene, polypropylene, fluoropolymer, and acetal homopolymer (see Table II).
Substrate | Ethyl Cyanoacrylate | Toughened Ethyl | Ethyl Cyanoacrylate |
Acetal | 200 | 100 | 1700 |
Fluoropolymer | 350 | 200 | 1050 |
Polyethylene | 150 | <50 | 500 |
Polypropylene | 50 | 50 | >1950 |
Table II. Cyanoacrylate block shear strength (in psi per ASTM 4501).
Typical medical device applications involving cyanoacrylates include the assembly of latex balloons and stainless-steel tips for catheters, and the assembly of tube sets.
Light-Curable Acrylics. When exposed to light of the appropriate wavelength and intensity, light-curable acrylic adhesives undergo a free-radical reaction to form thermoset resins. Like cyanoacrylates, light-curable acrylics range from low-viscosity formulations (~ 50 cP) to thixotropic gels. In addition, these adhesives vary in final cured form from resins that are hard and glasslike to those that are soft and flexible.
The critical processing key with light-curable acrylics is that light must reach the full bond line in order to cure the adhesive; any adhesive in shadowed areas will not cure. In addition, the maximum depth of cure for the majority of light-curable acrylic systems is approximately 0.5 in. A further consideration when selecting any light-curable adhesive is the equipment required for processing the product. Because these adhesives require a specific level of radiant energy for the polymerization reaction to occur, it is critical that end-users match the adhesive with the appropriate light source. Typical low-intensity systems can have an average price of $1000, while high-intensity, custom systems can run into the tens of thousands of dollars.
Light-curable acrylic adhesives offer the significant benefit of rapid fixturing and cure (as little as 5 seconds for select joints) following exposure, thus minimizing work in process. They are also designed to bond a wide variety of substrates and yield a clear bond line when used in thin sections. Because light-curable acrylics cure as thermoset plastics, they offer enhanced thermal, chemical, and environmental resistance compared with cyanoacrylate adhesives.
Among the numerous medical applications involving light-curable acrylic adhesives are needle assembly, anesthesia-mask bonding, polycarbonate component assembly (e.g., blood oxygenators, heat exchangers, and surgical pumps), and hearing-aid molding.
Light-Curable Cyanoacrylates. A new technology introduced in the United States in 1998 combines the benefits of cyanoacrylate and light-curable acrylic adhesives. Light-curable cyanoacrylates are ethyl-based products with photoinitiators added to the formulation. The end result is both fast fixturing—like that of a traditional light-curable acrylic—and cure in shadowed areas. Because light-curable cyanoacrylates are ethyl monomer based, their overall physical-performance characteristics are similar to those obtained with traditional cyanoacrylates. Additional benefits gained with the new technology include minimal blooming (since exposed, uncured cyanoacrylate can be immediately cured using ultraviolet and/or visible light), increased depth of cure compared with the traditional maximum cyanoacrylate cure depth of 0.010 in., and compatibility with primers for hard-to-bond plastics. Typical applications for light-curable cyanoacrylates are similar to those outlined for standard cyanoacrylates, with the added benefit of rapid cure in areas exposed to light.
Epoxies. Like light-curable acrylics, epoxy adhesives cure to form thermoset plastics. The polymerization reaction occurs via ring opening of an epoxide group initiated by a catalyst such as an amine or mercaptan. Both room-temperature and heat-curable one- and two-part systems are available. Because of their ability to cross-link, epoxies offer superior chemical, environmental, and thermal resistance. The fact that they can bond a variety of substrates and fill large gaps makes epoxies useful for deep-section potting of medical components and needle assembly.
Since epoxies cure via an exothermic reaction (giving off heat during cure), their use on temperature-sensitive components must be closely monitored. A second potential drawback to epoxy use is their rigid nature when cured, which can result in low peel strengths.
Polyurethanes. Polyurethane adhesives, like epoxies, come in one- and two-part formulations. A urethane linkage occurs when the two main components—a polyol and an isocyanate—react to form hard and soft segments in the resultant cured polymer, which make it uniquely flexible yet tough. Like several of the previously mentioned chemistries, polyurethane adhesives form thermoset resins when cured and exhibit good chemical and environmental resistance. It is important to note, however, that the overall thermal resistance of cured polyurethanes is less than that of cured epoxies.
Although polyurethane adhesives can be applied to a range of substrates, they occasionally mandate the use of a primer to increase the reactivity of the surface to be bonded. Many of these primers require long on-part times in order to effectively prepare the surface for the adhesive. A second potential drawback to the use of polyurethanes is their inherent sensitivity to moisture. Excess moisture on a part or in one of the adhesive components can cause a reaction that results in the evolution of carbon dioxide and the presence of bubbles in the finished part.
Common uses of polyurethanes in the medical device market include bonding tips on catheters and optical scopes, sealing oxygenators and heat exchangers, and assembling components that require significant flexibility.
Silicones. Similar to polyurethanes in that they form flexible polymers when cured, silicone adhesives possess no rigid segments and therefore exhibit lower cohesive strengths—that is, the ability of the polymer to adhere to itself. Like epoxies and polyurethanes, silicone adhesives are available in several forms, including one-part moisture cure; one-part heat cure; and one-part, dual moisture- and light-cure formulations. Although two-part industrial silicone systems do exist, the catalysts used in such materials typically cause them to fail biocompatibility screening.
The majority of moisture-cure silicones have two primary characteristics that limit their use in the medical device market: the evolution of by-products such as acetic acid, and a lengthy, 24-hour cure time. The use of dual curing systems—which react initially to light and subsequently to moisture—provide cure-on-demand fixture strength followed by full cure within 72 hours.
Typical silicone applications involve bonding and sealing of silicone-based assemblies, coating of components to minimize rough edges or burrs, and coating of highly flexible assemblies such as endotracheal and tracheotomy tubes.
A summary of the benefits and limitations of several of these adhesives is provided in Table III.
Chemistry | Benefits | Limitations | Typical Applications |
Cyanoacrylate | Substrate versatility | Thermoplastic resin | Catheter components |
Light-Curable | Substrate versatility | Capital expenditure | Needle assembly |
Epoxy | Substrate versatility | Poor peel strength, | Needle assembly |
Polyurethane | Substrate versatility | Moisture sensitivity | Deep section potting |
Table III. Summary of benefits, limitations, and typical applications of common medical device adhesives.
CONCLUSION
A wide variety of assembly methods are available to medical device designers and manufacturers. Among them, adhesives offer numerous benefits that cannot be obtained using traditional welding techniques. The variety of formulations that currently meet biocompatibility requirements ensure that the majority of device assembly applications can be quickly and effectively completed using adhesives.
Christine Salerni is an application engineering chemist at Loctite Corp. (Rocky Hill, CT). The company supplies an extensive range of adhesives to the medical device industry.
Return to the MDDI June table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like


