Here's why a medical device manufacturer may want to consider parylene conformal coatings for implantable devices.
April 19, 2017
Here's why a medical device manufacturer may want to consider parylene conformal coatings for implantable devices.
Sean Horn
The chemical vapor deposition (CVD) process is unique to Parylene compared to other common conformal coatings.
Parylene provides precisely deposited protective conformal coatings for medical implants, enabling the specific device purpose despite challenging physical configurations. Other performance properties amplify parylene's ability to withstand operational duress throughout surgical implantation and long-term use in the body:
dependable elastometricity,
functional stability in the presence of bodily fluids,
exceptional biocompatibility, and
outstanding hydrophobicity
Parylene films serve simultaneously as both a vehicle for drug delivery to specified zones within the body and a mechanism for controlled pharmaceutical release. The coatings provide implant protection that resists water and body sweat, as well as chemicals, corrosives, and solvents, protecting the device's function and performance whether worn on the body or implanted internally.
Parylene Deposition
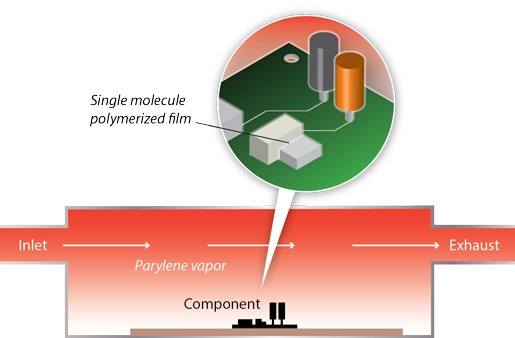
The chemical vapor deposition (CVD) process is unique to parylene compared to other common conformal coatings. The vapor deposition process used for parylene application generates a durable, pinhole-free conformal film on complex implant shapes, through a wide range of substrate/product materials, including ceramic, composite, and metallic substances. Liquid coatings such as acrylic, epoxy, silicone, and urethane use traditional methods for application to printed circuit boards--brushing, dipping, spraying, etc.--that can result in pooling, ridges, or an uneven coating. Vapor-based deposition assures parylene coatings are more truly conformal than liquid coatings.
Vapor-phase, chemical-vacuum polymerization converts chemically inert, powdered parylene dimer into a gaseous form at the molecular level. In the first stage of the deposition process, the powdered dimer precursor is heated under vacuum, initiating its transformation to a vapor. In a gaseous state, the parylene dimer is conveyed to the chamber's furnace and when treated at over 600°C, pyrolysis into a reactive monomer occurs. Lastly, the parylene gas deposits as a thin film in the deposition chamber. No catalyst or solvent is required to assure deposition of parylene.
The advantage of gaseous CVD application is ready penetration of even the smallest crevices, tightening all areas on multi-layer elements with a uniform coating of manageable thickness, adhering well to the performance requirements of the implant.
Parylene uses the Gorham Process, which minimizes the use and generation of hazardous substances. Parylene deposition avoids common drawbacks such as bridging and overfilming of minute surface openings, problems that frequently develop with wet coating materials. Enacted in a specialized vacuum chamber, the process enables parylene to coat the inside of seemingly closed structures, penetrating surface openings at the micron/individual molecule level. Parylene deposition completely and effectively encapsulates the device's external features with a resilient, protective film.
Protecting Medical Implants
Implantable medical devices are only as functional as the protection they receive. A wide range of implantable medical instruments profit from the application of parylene coatings. Among medical devices benefiting from parylene film protection are:
brain probes,
bone-growth stimulators,
bone pins,
cannulae,
cardiac assists devices (CADs),
catheters,
cochlear ear implants,
electronic circuits,
electrosurgical devices (ESU) instruments,
injection needles,
mandrels,
pneumatically-powered surgical instruments,
prosthetic components,
orthopedic devices of all kinds,
sensors for diagnostic monitoring of patients' conditions, and
ultrasonic transducers
Of these, a description of three--CADs (cardiac assist devices), cochlear implants, and sensor implants--exemplifies how parylene conformal films enable and enhance implantables' function.
Cardiac Assist Devices (CADS): The precise and complex medical electronics of implantable CADs like cardiac pacemakers and cardioverter-defibrillators (ICDs) benefit from ongoing protection by parylene's superior conformal coatings. Parylene seals these instruments, protecting them from the potentially corrosive impact of bodily fluids circulating in the heart's vicinity, stimulating and verifying the patient's regular heartbeat and otherwise enacting procedures that prevent cardiac failure or malfunction. Parylene-coated CADs effectively safeguard against bodily fluids and similar biocontaminants, assuring the CAD's electrical assemblies' ongoing (and necessary) function. This also minimizes the body's exposure to electrical charges or materials leaking from the implanted CAD, further protecting the patient.
|
Parylene conformal films can be used on cochlear implants. |
Cochlear ear implants: Inserted into the spiral cavity of the inner ear, cochlear implants stimulate production of nerve impulses in response to sound vibrations for people with hearing problems. The implant's electronics' package contains a series of conductors and electrodes along its length. The ear is a surprisingly caustic environment, high in sulfur content that can damage electronics. In current uses, a thin layer of parylene completely encapsulates the implant to prevent the sulfur and other contaminants from damaging the sensitive electronics. In some instances, an innovative flexible parylene-based electrode array fabricated as a vapor-deposited, micron-thick film offers sufficient patient protection, enhancing device longevity.
Sensor implants are useful for diagnostic monitoring of patients' conditions and ensuring medical devices are working as designed. Wherever they are situated in the body--in sites ranging from the bladder to the brain--sensors need to be coated with a suitably biocompatible material that does not damage the surrounding tissues, providing biostability, corrosion resistance, durability, enhanced lubricity, and surface consolidation to avert flaking or dusting. This promotes the longer-term functionality of the device. Sensor types that benefit from parylene conformal films include applications for:
electrochemical impedance spectroscopy (EIS), to classify cervical smears or urinary bladder infections;
microelectrode arrays for electromyography (EMG) or endoscopic equipment; and
surface acoustic waves (SAWs), used for oxygen plasma treatments or for detecting cell volume/antibody film levels.
In all cases, biomedical sensors and related hybrid flexible sensing platforms record real-time physiological activities of organs and tissue.
Conclusion
Parylene conformal coatings are highly biocompatible, generally integrating well in the human body. In addition to biocompatibility, parylene coatings are biostable, inert, non-toxic, and meet FDA compliance guidelines, increasing antimicrobial protection during extended use. MEMS/nano devices for diagnostic monitoring of patient conditions are aided by parylene's dependable, ultra-thin, secure conformal coating. Durable, insulating, pinhole-free parylene films conformally protect the implanted device, minimizing potential leakage into the bodily system during use, and protecting the patient from infection or irritation of the blood stream and internal organs.
Sean Horn is the chief financial officer at Diamond-MT, Inc. in Johnstown, PA. Diamond-MT specializes in parylene and conformal coating services.
[Images courtesy of DIAMOND-MT]
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)